Impaired capillary–venous drainage contributes to gliosis and demyelination in mouse white matter during aging
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
The progressive loss of cerebral white matter during aging contributes to cognitive decline, but whether reduced blood flow is a cause or a consequence remains debatable. Using deep multi-photon imaging in mice, we examined microvascular networks perfusing myelinated tissues in cortical layer 6 and the corpus callosum. We identified sparse, wide-reaching venules, termed principal cortical venules, which exclusively drain deep tissues and resemble the vasculature at the human cortex and U-fiber interface. Aging led to selective constriction and rarefaction of capillaries in deep branches of principal cortical venules. This resulted in mild hypoperfusion that was associated with microgliosis, astrogliosis and demyelination in deep tissues, but not the upper cortex. Induction of comparable hypoperfusion in adult mice using carotid artery stenosis triggered a similar tissue pathology specific to layer 6 and the corpus callosum. Thus, impaired capillary–venous drainage is a contributor to hypoperfusion and a potential therapeutic target for preserving blood flow to white matter during aging. How reduced blood flow plays a role in progressive white matter loss during aging and associated cognitive decline is unclear. Here the authors show that selective constriction and rarefaction of capillary–venous networks contribute to age-related hypoperfusion and white matter damage in mice.

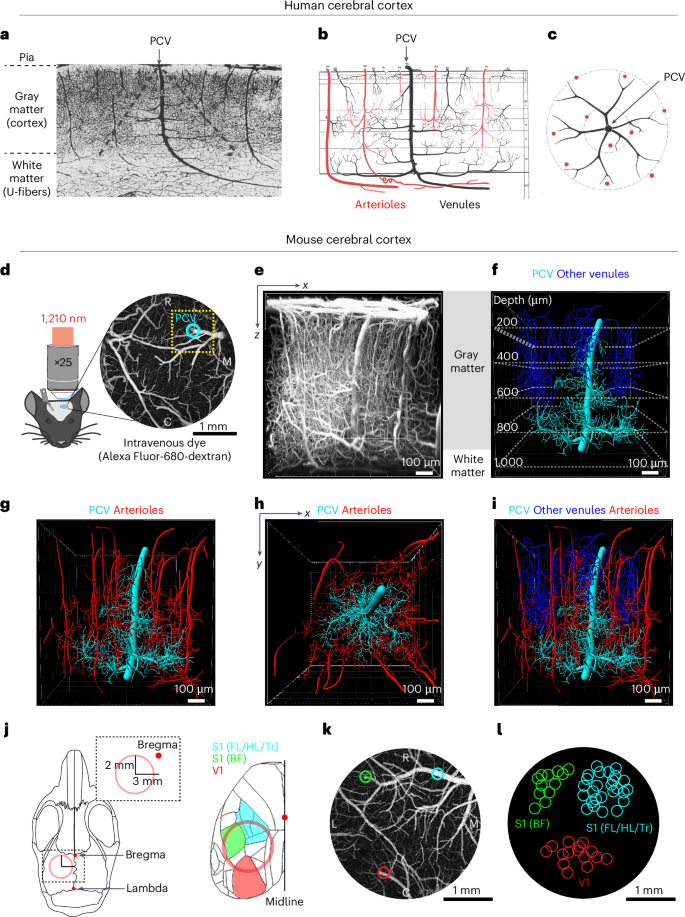
在衰老过程中,毛细血管-静脉引流受损导致小鼠白质胶质增生和脱髓鞘
随着年龄的增长,大脑白质的逐渐减少会导致认知能力下降,但血流量减少是原因还是结果仍有争议。利用深多光子成像技术,研究了小鼠皮层第6层髓鞘组织和胼胝体的微血管网络。我们发现了稀疏的、伸展的小静脉,称为皮层主小静脉,它们专门引流深层组织,类似于人类皮层和u -纤维界面的脉管系统。衰老导致皮质小静脉深部分支的毛细血管选择性收缩和稀疏。这导致轻度灌注不足,与深部组织的小胶质细胞增生、星形胶质细胞增生和脱髓鞘有关,但与上皮层无关。在成年小鼠中使用颈动脉狭窄诱导类似的灌注不足引发了类似的第六层和胼胝体特有的组织病理。因此,毛细血管-静脉引流受损是造成灌注不足的原因之一,也是在衰老过程中保持白质血流的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


