A STUB1–CHIC2 complex inhibits CD8+ T cells to restrain tumor immunity
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
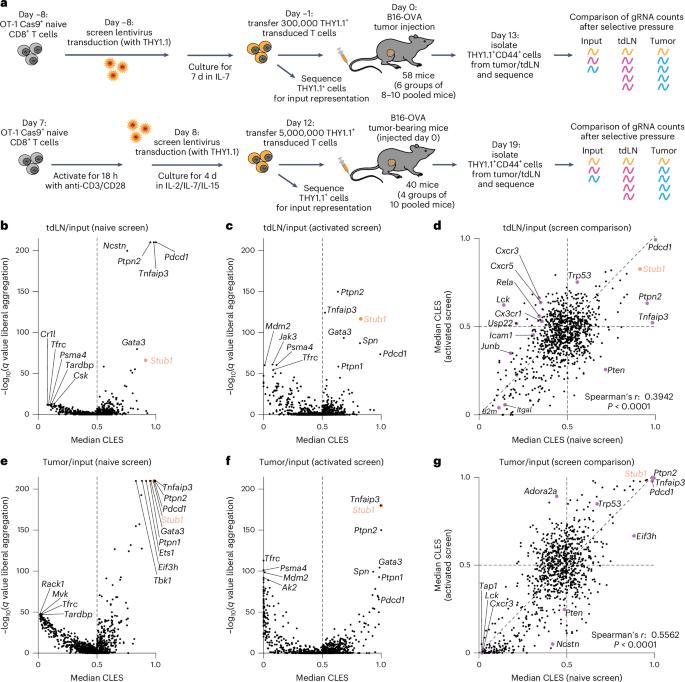
In vivo CRISPR screens in CD8+ T cells have previously uncovered targets for cancer immunotherapy; however, a minority of the genome has been individually annotated, suggesting that additional regulators remain to be discovered. Here we assessed 899 genes in CD8+ T cells responding to murine melanoma and identified the E3 ubiquitin ligase STUB1 as a new negative regulator of anti-tumor CD8+ T cell function. We demonstrated that Stub1 knockout CD8+ T cells effectively control tumor growth across multiple murine models. Mechanistically, STUB1 interacts with the adapter protein CHIC2 to regulate cytokine receptor expression in mouse and human CD8+ T cells. Among the regulated cytokine receptors, interleukin-27 receptor α is essential for tumor growth control mediated by Stub1/Chic2 knockout CD8+ T cells. Together, these findings establish the STUB1–CHIC2 complex as a regulator of cytokine receptor expression in CD8+ T cells and provide rationale for inhibiting this pathway to enhance CD8+ T cell-mediated anti-tumor immunity. LaFleur, Milling et al. perform an in vivo CRISPR screen of CD8+ T cells responding to tumors. They identify the E3 ubiquitin ligase STUB1 as a potent negative regulator of CD8+ T cell responses in tumors.
STUB1-CHIC2复合物抑制CD8+ T细胞抑制肿瘤免疫
CD8+ T细胞的体内CRISPR筛选先前已经发现了癌症免疫治疗的靶点;然而,基因组的一小部分已经被单独注释,这表明更多的调节因子仍有待发现。在这里,我们评估了899个CD8+ T细胞中响应小鼠黑色素瘤的基因,并确定E3泛素连接酶STUB1是抗肿瘤CD8+ T细胞功能的新的负调节因子。我们证明了敲除Stub1的CD8+ T细胞可以有效地控制多种小鼠模型的肿瘤生长。在机制上,STUB1与适配蛋白CHIC2相互作用,调节小鼠和人CD8+ T细胞中细胞因子受体的表达。在这些受调控的细胞因子受体中,白细胞介素-27受体α (interleukin-27 receptor α)在Stub1/Chic2敲除CD8+ T细胞介导的肿瘤生长控制中起重要作用。总之,这些发现证实了STUB1-CHIC2复合物是CD8+ T细胞中细胞因子受体表达的调节因子,并为抑制该通路以增强CD8+ T细胞介导的抗肿瘤免疫提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


