TNF and type I interferon crosstalk controls the fate and function of plasmacytoid dendritic cells
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
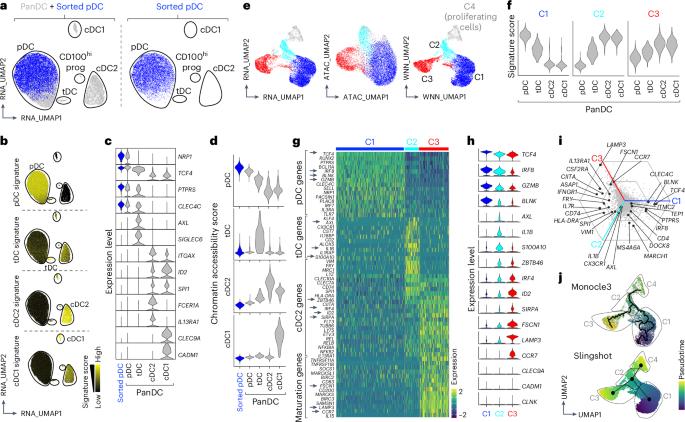
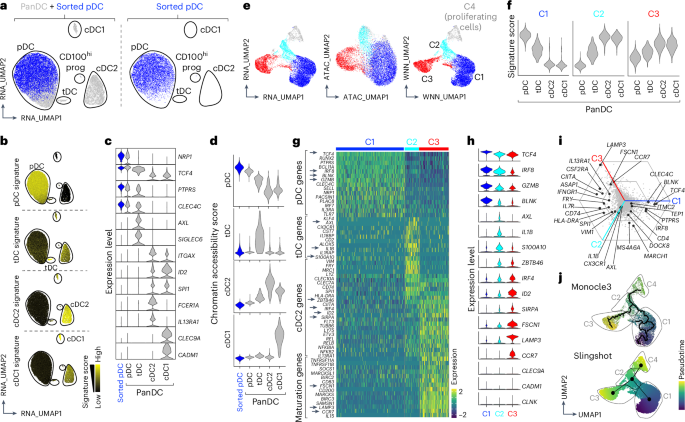
Plasmacytoid dendritic cells (pDCs) are major producers of type I interferon (IFN-I), an important antiviral cytokine, and activity of these cells must be tightly controlled to prevent harmful inflammation and autoimmunity. Evidence exists that one regulatory mechanism is a fate-switching process from an IFN-I-secreting pDC to a professional antigen-presenting conventional dendritic cell (cDC) that lacks IFN-I-secreting capacity. However, this differentiation process is controversial owing to limitations in tracking the fate of individual cells over time. Here we use single-cell omics and functional experiments to show that activated human pDCs can lose their identity as IFN-I-secreting cells and acquire the transcriptional, epigenetic and functional features of cDCs. This pDC fate-switching process is promoted by tumor necrosis factor but blocked by IFN-I. Importantly, it occurs in vivo during human skin inflammatory diseases and injury, and physiologically in elderly people. This work identifies the pDC-to-cDC reprogramming trajectory and unveils a mechanistic framework for harnessing it therapeutically. Plasmacytoid dendritic cells are type 1 interferon (IFN-I)-producing antiviral specialists that have been shown to be able to differentiate into conventional dendritic cells. Here the authors show how this differentiation is controlled by tumor necrosis factor driving type 2 conventional dendritic cell-like reprogramming and IFN-I blocking it, a process that occurs during inflammation, injury and aging.
TNF和I型干扰素串扰控制浆细胞样树突状细胞的命运和功能
浆细胞样树突状细胞(pDCs)是I型干扰素(IFN-I)的主要产生者,IFN-I是一种重要的抗病毒细胞因子,必须严格控制这些细胞的活性,以防止有害的炎症和自身免疫。有证据表明,从分泌ifn - i的pDC到缺乏分泌ifn - i能力的专业抗原呈递常规树突状细胞(cDC)的命运转换过程是一种调控机制。然而,这种分化过程是有争议的,因为跟踪单个细胞的命运随时间的限制。本研究利用单细胞组学和功能实验证明,活化的人pDCs可以失去ifn - i分泌细胞的身份,并获得cdc的转录、表观遗传和功能特征。这种pDC命运转换过程由肿瘤坏死因子促进,但被IFN-I阻断。重要的是,它发生在人体皮肤炎症性疾病和损伤期间,也发生在老年人的生理上。这项工作确定了pdc到cdc的重编程轨迹,并揭示了利用它进行治疗的机制框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


