Purification, characterization and the hemolytic mechanism of the hemolysin NnTX-45 from the jellyfish Nemopilema nomurai
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Nemopilema nomurai, a large venomous jellyfish, caused numerous stinging incidents and even many fatal cases. The venom of N. nomurai contains various toxins, with hemolysin being one of the major components that play a crucial role in stinging. However, until now, the hemolysin has not been successfully isolated from N. nomurai, and its mechanism remains unclear. In this study, we established an improved method for preparing jellyfish toxins to facilitate subsequent purification. The SDS-PAGE profiles indicated that the crude toxin extracted using this improved method contained significantly fewer components than the traditional method. Moreover, the proteomic and toxicity analysis revealed that the refined extract still contained most of the key toxins including hemolysin, phospholipase, and other toxins. The hemolysin was then isolated from the refined jellyfish crude toxins using a two-step purification of gel filtration chromatography followed by anion-exchange chromatography. The molecular mass of this hemolysin was 45 kDa (NnTX-45) with an HC50 of approximately 30 μg/mL. Transmission electron microscopy observations revealed that NnTX-45 formed numerous pores, each with an inner diameter of 5.65 nm and an outer diameter of 13 nm approximately, on the erythrocyte membranes. Overall, our study successfully isolated and elucidated the preliminary hemolytic mechanism of the NnTX-45 from N. nomurai, which provides a highly purified toxin antigens for the development of jellyfish antivenom to treat this jellyfish sting in the future.
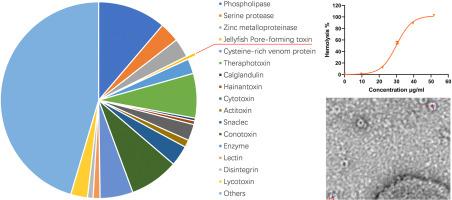
水母溶血素NnTX-45的纯化、表征及溶血机制研究。
野水母是一种巨大的有毒水母,它造成了许多蜇伤事件,甚至造成了许多致命的病例。野村N.的毒液含有多种毒素,溶血素是在刺痛中起关键作用的主要成分之一。然而,到目前为止,溶血素尚未从野乳杆菌中成功分离出来,其作用机制尚不清楚。在本研究中,我们建立了一种改进的水母毒素制备方法,便于后续的纯化。SDS-PAGE图谱表明,改进后的方法所提取的粗毒素成分明显少于传统方法。此外,蛋白质组学和毒性分析表明,精制提取物仍含有大部分关键毒素,包括溶血素、磷脂酶等毒素。通过凝胶过滤层析和阴离子交换层析两步纯化,从海蜇粗毒素中分离出溶血素。该溶血素分子量为45 kDa (NnTX-45), HC50约为30 μg/mL。透射电镜观察发现,NnTX-45在红细胞膜上形成了许多孔,每个孔的内径约为5.65 nm,外径约为13 nm。总之,本研究成功分离并初步阐明了NnTX-45的溶血机制,为今后开发水母抗蛇毒血清治疗水母蜇伤提供了一种高纯度的毒素抗原。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


