Molecular and neural control of social hierarchy by a forebrain-thalamocortical circuit
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Many animal groups are organized hierarchically, which generates behavioral states that facilitate social interactions. Although generally stable, social status can change, underscoring the plasticity of underlying neural circuits. We examined competition among unfamiliar male mice and uncovered how the molecular and biophysical characteristics of a forebrain-thalamocortical circuit affect hierarchy. We identify the mediodorsal thalamus (MDT) as a hub receiving inputs from the orbitofrontal cortex and basal forebrain and projecting to the caudal anterior cingulate cortex (cACC) to regulate competitive performance. This circuit becomes potentiated or depressed in high- and low-rank males, respectively, in part through altered expression of the voltage-gated ion channel Trpm3 and synaptic plasticity. In high-rank mice, MDT projections drive inhibition of cACC pyramidal cells, promoting winning, in a pattern strikingly opposite to the dorsomedial prefrontal cortex, where winners display increased pyramidal cell activity. Our data suggest a model in which hierarchy modulation relies on coordinated remodeling of multiple forebrain-thalamocortical circuits.
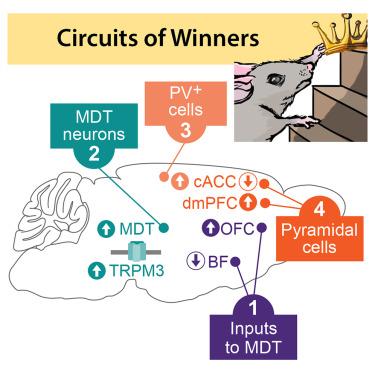
前脑-丘脑皮质回路对社会等级的分子和神经控制
许多动物群体都是按等级组织的,这就产生了促进社会互动的行为状态。虽然社会地位通常是稳定的,但它可以改变,这强调了潜在神经回路的可塑性。我们研究了不熟悉的雄性小鼠之间的竞争,并揭示了前脑-丘脑皮质回路的分子和生物物理特征如何影响等级。我们确定丘脑中背侧(MDT)是一个中枢,接收来自眶额皮质和基底前脑的输入,并投射到尾侧前扣带皮层(cACC),以调节竞争表现。这一回路在高级别雄性和低级别雄性中分别增强或抑制,部分原因是电压门控离子通道Trpm3和突触可塑性的表达改变。在高级别小鼠中,MDT投射驱动cACC锥体细胞的抑制,促进获胜,其模式与背内侧前额叶皮层截然相反,在那里,获胜者显示锥体细胞活性增加。我们的数据提出了一个模型,其中层次调节依赖于多个前脑-丘脑皮质回路的协调重塑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


