Differential kinematic coding in sensorimotor striatum across behavioral domains reflects different contributions to movement
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
The sensorimotor arm of the basal ganglia is a major part of the mammalian motor control network, yet whether it supports all movements or is specialized for task-oriented behaviors remains unclear. To examine this, we probed the contributions of the rat sensorimotor striatum (dorsolateral striatum (DLS)) in two behavioral domains: free exploration, in which naturalistic behaviors are expressed, and during a motor task. In contrast to prior work, which showed the DLS being essential for generating task-specific learned movements, DLS lesions had no effect on naturalistic behaviors like rearing, grooming or walking. To explore the neural basis of this functional dissociation, we compared DLS activity across the two domains. Although neural activity reflected movement kinematics in both, the kinematic codes differed starkly. These findings suggest that sensorimotor basal ganglia are not essential parts of mammalian motor control but, rather, shift their output into a motor-potent space to shape task-specific behaviors. Hardcastle and Marshall et al. show that striatal function is domain specific, required for task-related but not spontaneously expressed movements. This functional distinction is reflected in starkly different kinematic codes across the domains.
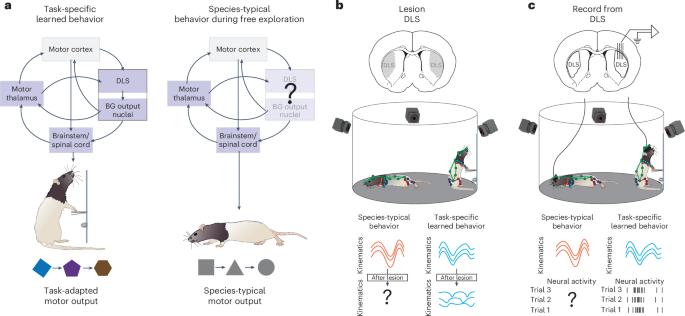
不同行为域的感觉运动纹状体的不同运动编码反映了对运动的不同贡献
基底神经节的感觉运动臂是哺乳动物运动控制网络的主要部分,但它是否支持所有运动或专门用于任务导向行为尚不清楚。为了检验这一点,我们探讨了大鼠感觉运动纹状体(背外侧纹状体(DLS))在两个行为领域的贡献:自由探索,自然行为的表达和运动任务。先前的研究表明,DLS对于产生特定任务的学习动作至关重要,与此相反,DLS病变对养育、梳理或行走等自然行为没有影响。为了探索这种功能分离的神经基础,我们比较了两个区域的DLS活动。尽管神经活动反映了两者的运动运动学,但运动学编码明显不同。这些发现表明,感觉运动基底神经节不是哺乳动物运动控制的必要部分,而是将其输出转移到运动有效空间,以形成特定任务的行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


