Sex differences in resilience at puberty depend upon divergent effects of a stress steroid at α4βδ GABAA receptors
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
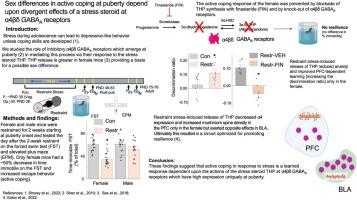
Resilience is a critical skill that lessens the adverse effects of stress which are increasingly reported in adolescents, where sex differences are noted. It is not known how this process develops in adolescence when unique changes in neuronal properties occur. For this study, pubertal mice were tested for their coping ability following 2-week restraint. We show here that this predictable stress produced resilience in pubertal female mice where time immobile in the forced swim test (FST) decreased by ∼50 % (P < 0.0001), an effect that extended into adulthood, and increased escape behavior 8-fold (P = 0.01). This effect was not seen in pubertal male or adult female mice. This process required the stress steroid 3α-OH,5α-pregnan-20-one (THP, allopregnanolone) and its primary target, α4βδ GABAA receptors (GABARs). These receptors emerge at puberty in prelimbic prefrontal cortex (PL PFC) and basolateral amygdala (BLA), which play a pivotal role in resilient behavior. Stress-induced release of THP decreased anxiety-like behavior (increasing open arm time in the elevated plus maze) and enhanced PFC-dependent learning (temporal order recognition) after 1d restraint in pubertal female, but not male, mice while differentially altering α4βδ expression in PL and BLA. This divergent THP-induced effect ultimately increased and decreased mushroom spine density in PL and BLA, respectively, to produce a circuit optimized for resilient behavior in the pubertal females. These findings demonstrate a novel mechanism for the development of resilience unique to the pubertal period. The results from the present study may suggest therapeutic strategies for adolescent stress which would impact mental health in adulthood.
青春期恢复力的性别差异取决于应激类固醇对α4βδ GABAA受体的不同作用。
适应力是一项关键技能,可以减轻压力带来的不利影响,这种影响越来越多地出现在青少年身上,其中性别差异尤为明显。目前尚不清楚这一过程在青少年时期是如何发展的,当时神经元特性发生了独特的变化。在本研究中,我们测试了青春期小鼠在2周的约束后的应对能力。我们在这里表明,这种可预测的压力在青春期雌性小鼠中产生了弹性,在强迫游泳测试(FST)中固定时间减少了 ~ 50% % (P A受体(gabar))。这些受体在青春期出现在前边缘前额叶皮层(PL PFC)和基底外侧杏仁核(BLA)中,在弹性行为中起着关键作用。应激诱导的THP释放降低了青春期雌性小鼠的焦虑样行为(在高架+迷宫中增加张开手臂的时间),增强了pfc依赖性学习(时间顺序识别),而雄性小鼠则没有,同时不同程度地改变了PL和BLA中α4βδ的表达。这种发散的thp诱导效应最终分别增加和减少了PL和BLA中的蘑菇棘密度,从而产生了一个优化了青春期雌性弹性行为的回路。这些发现证明了青春期特有的韧性发展的新机制。本研究的结果可能为青少年压力的治疗策略提供建议,这些压力可能会影响成年期的心理健康。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


