Global landscape of PROTAC: Perspectives from patents, drug pipelines, clinical trials, and licensing transactions
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Proteolysis-targeting chimeras (PROTACs) represent an emerging therapeutic strategy that has experienced rapid growth since the first clinical proof-of-concept in 2020. Although numerous technical reviews exist, systematic analyses integrating multidimensional data from discovery through commercialization remain scarce. To fill this gap, this study analyzed comprehensive datasets from 2004 to 2024, covering 4840 patent applications, 170 drug pipelines, 123 clinical trials, and 44 licensing transactions, characterizing global trends, geographic distributions, collaborations, therapeutic landscapes, key targets, and stakeholder dynamics. From 2023 to 2024, clinical trials increased by 57 %, patent families by 28 %, collaborative patents by 87 %, and new patent entrants by 28 %. The U.S. and China dominate global PROTAC competition, with the U.S. leading in patent strength and new entrants, and China advancing rapidly in clinical translation and early-stage pipelines. Although licensing activities initially peaked in 2020, a rationalized decline followed. PROTAC targets have diversified from classical oncology proteins to include previously “undruggable” oncology, immunology, and neurology targets. Among the top 15 patent assignees by patent family count, 11 are pharmaceutical companies, of which 9 have advanced at least one clinical-stage PROTAC pipeline candidate into global leadership, although some programs have since been discontinued. This study fills a critical gap by providing systematic competitive intelligence analysis, complementing existing PROTAC reviews that mainly address molecular mechanisms and technical challenges. By integrating patents, drug pipelines, clinical trials and licensing transactions, this study offers broader industrial and strategic perspectives, effectively supplementing current fundamental scientific literature to collectively establish a more comprehensive understanding of the PROTAC field.
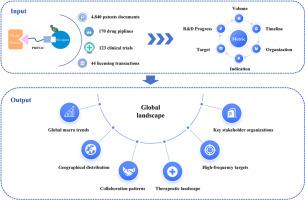
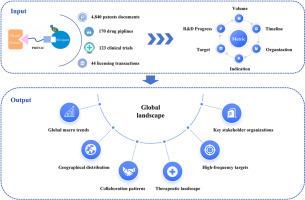
PROTAC的全球前景:从专利、药物管道、临床试验和许可交易的视角
自2020年首次临床概念验证以来,靶向蛋白水解嵌合体(PROTACs)代表了一种新兴的治疗策略,经历了快速增长。尽管存在大量的技术审查,但整合从发现到商业化的多维数据的系统分析仍然很少。为了填补这一空白,本研究分析了2004年至2024年的综合数据集,涵盖了4,840项专利申请、170种药物管道、123项临床试验和44项许可交易,并描述了全球趋势、地理分布、合作、治疗前景、关键目标和利益相关者动态。从2023年到2024年,临床试验增加了57%,专利家族增加了28%,合作专利增加了87%,新专利进入者增加了28%。美国和中国主导着全球PROTAC竞争,美国在专利实力和新进入者方面处于领先地位,中国在临床转化和早期管道方面发展迅速。尽管许可活动最初在2020年达到顶峰,但随后出现了合理的下降。PROTAC的靶点已经从传统的肿瘤蛋白多样化到包括以前“不可药物”的肿瘤学、免疫学和神经学靶点。在专利家族数量排名前15位的专利受让人中,有11家是制药公司,其中9家已经将至少一个临床阶段的PROTAC候选药物推向全球领先地位,尽管有些项目已经停止。本研究通过提供系统的竞争情报分析填补了一个关键的空白,补充了现有的主要解决分子机制和技术挑战的PROTAC综述。通过整合专利、药物管道、临床试验和许可交易,本研究提供了更广阔的产业和战略视角,有效补充了现有的基础科学文献,共同建立了对PROTAC领域更全面的了解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


