Nanoparticle-induced excessive mitophagy combined with immune checkpoint blockade for enhanced cancer immunotherapy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Tumor microenvironment (TME) is the major obstacle in cancer immunotherapy due to its adverse effects on tumor-infiltrating immune cells. Emerging evidences have revealed that mitophagy plays an important role in regulating cell fate and immune microenvironment. Targeted regulation of mitophagy could be a promising strategy for enhanced cancer immunotherapy, which however remains unexploited due to the absence of robust therapeutic platform. We herein developed a mitophagy-induced RNA interfering (RNAi) nanoplatform composed of a hydrophilic polyethylene glycol (PEG) shell and an endosomal pH-responsive hydrophobic core encapsulating the complexes of mitophagy-inducer carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and small interfering RNA (siRNA) for enhanced breast cancer (BCa) immunotherapy. Using the orthotopic and metastatic BCa tumor models, we demonstrate that this nanoplatform could effectively induce excessive mitophagy in BCa cells to suppress their proliferation and silence PD-L1 expression to block its immunosuppressive effect on CD8+ T cells. More importantly, excessive mitophagy could inhibit C![]() C motif chemokine ligand 2 (CCL2) secretion from BCa cells and thus alleviate the immunosuppressive effect on CD8+ T cells via impairing the tumor infiltration of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), which could ultimately combine with the PD-L1 silencing to synergistically enhance the antitumor immunity and inhibit BCa tumor growth.
C motif chemokine ligand 2 (CCL2) secretion from BCa cells and thus alleviate the immunosuppressive effect on CD8+ T cells via impairing the tumor infiltration of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), which could ultimately combine with the PD-L1 silencing to synergistically enhance the antitumor immunity and inhibit BCa tumor growth.
Statement of significance
Amplification of mitophagy in tumor cells has been considered as a promising strategy for effective cancer therapy due to its important role in regulating cell fate and TME. We herein developed a mitophagy-induced RNAi nanoplatform, which could effectively induce BCa cell death via amplifying mitophagy and enhance the tumoricidal ability of CD8+ T cells via silencing PD-L1 expression. More importantly, this nanoplatform-induced excessive mitophagy could inhibit tumor-derived CCL2 secretion and thus remodel the immunosuppressive TME via impairing the tumor infiltration of TAMs, Tregs, and MDSCs, leading to enhanced antitumor immunity and significant inhibition of BCa tumor growth. The nanoplatform developed herein could be used as an effective tool for enhanced cancer immunotherapy.
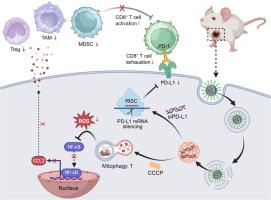
纳米颗粒诱导的过度线粒体自噬联合免疫检查点阻断增强癌症免疫治疗。
肿瘤微环境(Tumor microenvironment, TME)因其对肿瘤浸润性免疫细胞的不利影响而成为肿瘤免疫治疗的主要障碍。新的证据表明,有丝自噬在调节细胞命运和免疫微环境中起着重要作用。靶向调节线粒体自噬可能是增强癌症免疫治疗的一种有前途的策略,但由于缺乏强大的治疗平台,这一策略尚未得到开发。我们在此开发了一种线粒体自噬诱导的RNA干扰(RNAi)纳米平台,该平台由亲水性聚乙二醇(PEG)外壳和内体ph响应的疏水性核心组成,该核心包裹了线粒体自噬诱导剂羰基氰化物3-氯苯腙(CCCP)和小干扰RNA (siRNA)复合物,用于增强乳腺癌(BCa)免疫治疗。通过原位和转移性BCa肿瘤模型,我们证明该纳米平台可以有效诱导BCa细胞过度自噬以抑制其增殖,并沉默PD-L1的表达以阻断其对CD8+ T细胞的免疫抑制作用。更重要的是,过度自噬可以抑制BCa细胞分泌C-C基序趋化因子配体2 (CCL2),从而通过损害肿瘤相关巨噬细胞(tam)、调节性T细胞(Tregs)和髓源性抑制细胞(MDSCs)的肿瘤浸润来减轻对CD8+ T细胞的免疫抑制作用,最终可能与PD-L1沉默联合,协同增强抗肿瘤免疫,抑制BCa肿瘤生长。意义声明:肿瘤细胞中有丝分裂的扩增由于其在调节细胞命运和TME中的重要作用而被认为是一种有前途的有效癌症治疗策略。我们开发了一个线粒体自噬诱导的RNAi纳米平台,该平台可以通过扩增线粒体自噬有效诱导BCa细胞死亡,并通过沉默PD-L1表达增强CD8+ T细胞的杀瘤能力。更重要的是,这种纳米平台诱导的过度自噬可以抑制肿瘤来源的CCL2分泌,从而通过损害TAMs、Tregs和MDSCs的肿瘤浸润来重塑免疫抑制的TME,从而增强抗肿瘤免疫,显著抑制BCa肿瘤生长。本文开发的纳米平台可作为增强癌症免疫治疗的有效工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


