Dynamic release kinetics and biological impact of leachables from 3D-printed oral devices: An integrated in vitro and computational exposure model
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
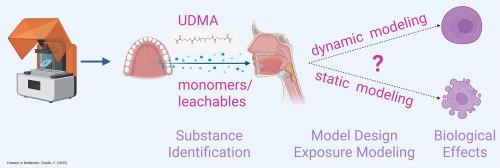
Research has highlighted the release of monomers and leachables from additively manufactured (AM) oral devices, raising concerns about their potential biological impact. The oral cavity's dynamic epithelial system necessitates exposure models that accurately reflect real-world conditions. Traditional static models often overestimate or underestimate patient exposure, failing to predict in vivo risks effectively. To address this gap, we developed an advanced dynamic oral tissue exposure model that simulates the release kinetics of leachables, saliva flow, and gingival tissue perfusion. This dynamic approach, integrated with an in vitro human gingival keratinocyte (HGK) model, was applied for the first time in this study. We quantified urethane dimethacrylate (UDMA) release from AM biomaterials through extraction experiments, generating data for computational modeling. The model revealed that dynamic in vivo monomer exposure peaks at specific time points before declining, a pattern not captured by static calculations. In vitro analysis showed that UDMA exposure inhibited metabolic activity and reduced Ki-67 expression in HGK cultures at micromolar concentrations. While inhibitory in vitro concentrations exceeded predicted in vivo estimates, low-dose effects on Ki-67 expression were still observed. These findings suggest that although calculated UDMA exposure remains sub-cytotoxic, it may still induce sensitizing effects. Overall, the dynamic exposure model introduced in this study represents a significant advancement in risk assessment, offering more accurate predictions of the biological effects of leachables and contributing to the safety evaluation of AM biomaterials.
Statement of significance
Additively manufactured (AM) oral devices are a significant source of monomer release into the oral cavity, raising concerns about tissue exposure. Traditional static models provide limited or inaccurate risk estimates due to the cavity’s dynamic nature. In this study, we developed a dynamic oral tissue exposure model that estimates in vivo-relevant monomer and leachable concentrations in saliva and oral mucosa while integrating an in vitro gingival keratinocyte model to assess biological effects. The model provides key insights into predicted in vivo exposure to monomers and leachables, improving in vitro evaluations of biological effects. Overall, it serves as a valuable risk assessment tool for the research community by enhancing predictions of patient exposure to potential monomers and leachables, thereby supporting AM biomaterial safety.
3d打印口腔设备中可浸出物的动态释放动力学和生物影响:一个集成的体外和计算暴露模型。
研究强调了增材制造(AM)口腔器械中单体和可浸出物的释放,引起了对其潜在生物影响的担忧。口腔的动态上皮系统需要准确反映真实情况的暴露模型。传统的静态模型往往高估或低估患者暴露,无法有效预测体内风险。为了解决这一差距,我们开发了一种先进的动态口腔组织暴露模型,模拟浸出物的释放动力学,唾液流动和牙龈组织灌注。该动态方法与体外人牙龈角质形成细胞(HGK)模型相结合,首次应用于本研究。我们通过提取实验量化了AM生物材料中UDMA的释放量,为计算模型生成数据。该模型显示,动态体内单体暴露在特定时间点达到峰值,然后下降,这是静态计算无法捕获的模式。体外分析表明,UDMA暴露在微摩尔浓度下抑制HGK培养物的代谢活性并降低Ki-67的表达。虽然体外抑制浓度超过体内预测,但仍观察到低剂量对Ki-67表达的影响。这些发现表明,虽然计算出的UDMA暴露仍然是亚细胞毒性的,但它仍然可能诱发致敏效应。总的来说,本研究中引入的动态暴露模型在风险评估方面取得了重大进展,可以更准确地预测浸出物的生物学效应,并有助于AM生物材料的安全性评估。意义声明:增材制造(AM)口腔器械是单体释放到口腔的重要来源,引起了对组织暴露的担忧。由于空腔的动态特性,传统的静态模型提供的风险估计有限或不准确。在这项研究中,我们开发了一个动态口腔组织暴露模型,该模型估计了唾液和口腔粘膜中与体内相关的单体和可浸出浓度,同时结合了一个体外牙龈角质细胞模型来评估生物效应。该模型为预测体内暴露于单体和可浸出物提供了关键见解,改善了体外生物效应评估。总体而言,它可以作为研究界有价值的风险评估工具,通过增强对患者暴露于潜在单体和浸出物的预测,从而支持AM生物材料的安全性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


