Patient-Derived 3D-Bioprinted Models of Pancreatic Cancer: Toward Personalized Therapy and Overcoming Tumor Microenvironment Challenges
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and treatment-resistant solid tumors, mainly due to its complex tumor microenvironment (TME). Characterized by dense desmoplastic stroma, immune suppression, and metabolic rewiring, the TME impairs drug delivery and eventually leads to therapeutic failure. Conventional models, such as two-dimensional (2D) cultures and mouse models, fail to recapitulate the cellular and mechanical intricacies of PDAC, limiting their translational relevance.
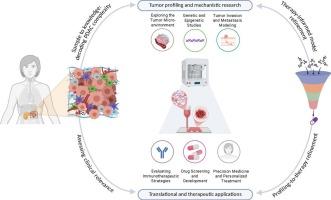
Three-dimensional (3D) models have emerged as promising tools to better simulate tumor biology. Among them, 3D-bioprinting techniques enable the precise spatial organization of cancer, stromal, and immune cells within tailored bioinks, supporting physiologically relevant architectures and dynamic microenvironmental interactions. These platforms allow controlled incorporation of extracellular matrix components, tunable stiffness, and perfusable vasculature, improving model fidelity and enabling real-time assessment of drug penetration, immune infiltration, and therapy resistance.
This review provides a comprehensive overview of current 3D PDAC modeling strategies, emphasizing patient-derived 3D-bioprinted models. We explore the key roles of bioink composition, extracellular matrix (ECM) stiffness, perfusion capacity, and immuno-compatibility in shaping the fidelity and utility of these models. Furthermore, we compare the structural complexity, scalability, drug-screening capabilities, and applicability for personalized medicine of different 3D models. By highlighting advances in vascularization, immune co-culture, and biofabrication technologies, we underscore the emerging value of 3D-bioprinting as a transformative platform for preclinical PDAC research. Ultimately, 3D-bioprinting is an important step forward in bridging the gap between preclinical studies and clinical implementation, as it opens the door to more accurate, personalized, and efficient therapeutic approaches for PDAC.
胰腺癌患者衍生的3d生物打印模型:个性化治疗和克服肿瘤微环境挑战
胰腺导管腺癌(Pancreatic ductal adenocarcinoma, PDAC)是侵袭性最强、最难治疗的实体肿瘤之一,主要是由于其复杂的肿瘤微环境(tumor microenvironment, TME)。TME以致密的间质、免疫抑制和代谢重组为特征,损害药物传递并最终导致治疗失败。传统的模型,如二维培养和小鼠模型,不能概括PDAC的细胞和机械复杂性,限制了它们的翻译相关性。三维(3D)模型已经成为更好地模拟肿瘤生物学的有前途的工具。其中,3d生物打印技术能够在定制的生物墨水中精确地组织癌症、基质和免疫细胞,支持生理相关架构和动态微环境相互作用。这些平台可以控制细胞外基质成分的掺入,调节刚度和可灌注血管,提高模型保真度,并能够实时评估药物渗透、免疫浸润和治疗耐药性。这篇综述提供了当前3D PDAC建模策略的全面概述,强调患者衍生的3D生物打印模型。我们探讨了生物链接成分、ECM刚度、灌注能力和免疫相容性在塑造这些模型的保真度和实用性中的关键作用。此外,我们比较了不同3D模型的结构复杂性、可扩展性、药物筛选能力和个性化医疗的适用性。通过强调血管化、免疫共培养和生物制造技术的进步,我们强调了3d生物打印作为临床前PDAC研究的变革性平台的新兴价值。最终,3d生物打印是弥合临床前研究和临床实施之间差距的重要一步,因为它为PDAC的更准确,个性化和有效的治疗方法打开了大门。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


