Study on the mechanism of two-dimensional heterostructured interlayer-confined catalysts for efficient PMS activation in antibiotic degradation: Interface engineering and 1O2 selective dominant pathway
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
To address the low efficiency and poor stability of advanced oxidation processes (AOPs), we developed a MXene-supported multilayered nanoreactor through interfacial engineering. By leveraging a bimetallic ZIF-derived topological transformation, we carefully engineered the CoZn-LDH/MXene heterointerface. This resulted in a two-dimensional heterostructure catalyst (CZLDH-MXene) with enhanced catalytic performance and stability. This 2D heterostructure optimizes the activation pathway, accelerates reaction kinetics, and enhances environmental adaptability. These improvements address the current limitations of persulfate-based advanced oxidation systems, offering a more efficient solution. Electrons are transferred from the catalyst to peroxomonosulfate (PMS) through the dual channels of Ti(II)/Ti(III)↔Ti(IV) and Co(II) ↔ Co(III), forming a stable CZLDH-MXene-PMS* interface complex, which proves the effectiveness of electron transfer process II mechanism (ETP II mechanism). The CZLDH-MXene/PMS system achieved complete degradation of 20 mg/L oxytetracycline (OTC) within 10 min. It also demonstrated broad pH tolerance (4–10) and resistance to ion interference. Quenching experiments and electron paramagnetic resonance (EPR) spectroscopy confirmed that the 2D heterostructure shifted the reaction mechanism from a radical-based pathway (•OH/SO4•−) to a singlet oxygen (1O2)-dominated non-radical mechanism, with 1O2 contributing 84.2 % to the process. The continuous flow reactor developed in this study showed stable and dynamic removal of simulated wastewater for up to 5 h, maintaining >98 % efficiency. This innovation overcomes the limitations of traditional batch reactors. These findings highlight the potential of this approach for water pollution control and introduce a new paradigm in designing efficient heterogeneous catalysts.
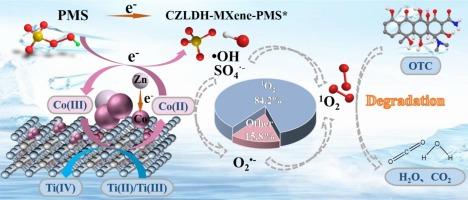
二维异质结构层间限制催化剂在抗生素降解中高效激活PMS的机理研究:界面工程和1O2选择优势途径
为了解决先进氧化工艺(AOPs)效率低、稳定性差的问题,我们通过界面工程开发了一种mxene负载的多层纳米反应器。通过利用双金属zif衍生的拓扑转换,我们精心设计了CoZn-LDH/MXene异质界面。这导致了二维异质结构催化剂(CZLDH-MXene)具有增强的催化性能和稳定性。这种二维异质结构优化了激活途径,加速了反应动力学,增强了环境适应性。这些改进解决了目前基于过硫酸盐的高级氧化系统的局限性,提供了一个更有效的解决方案。电子通过Ti(II)/Ti(III)双通道↔Ti(IV)和Co(II)↔Co(III)从催化剂转移到过氧化物单硫酸根(PMS)上,形成稳定的CZLDH-MXene-PMS*界面复合物,证明了电子转移过程II机制(ETP II机制)的有效性。CZLDH-MXene/PMS系统在10 min内实现了20 mg/L土霉素(OTC)的完全降解。它还表现出广泛的pH耐受性(4-10)和抗离子干扰。猝灭实验和电子顺磁共振(EPR)谱分析证实,二维异质结构将反应机理从基于自由基(•OH/SO4•−)的途径转变为以氧(1O2)为主的单线态非自由基机制,其中1O2对反应的贡献为84.2 %。本研究开发的连续流反应器对模拟废水的去除率高达5 h,保持98% %的效率。这种创新克服了传统间歇式反应器的局限性。这些发现突出了这种方法在水污染控制方面的潜力,并为设计高效的多相催化剂提供了新的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


