TCF19 drives a broad transcriptional program that potentiates optimal innate and adaptive functions of antiviral NK cells
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
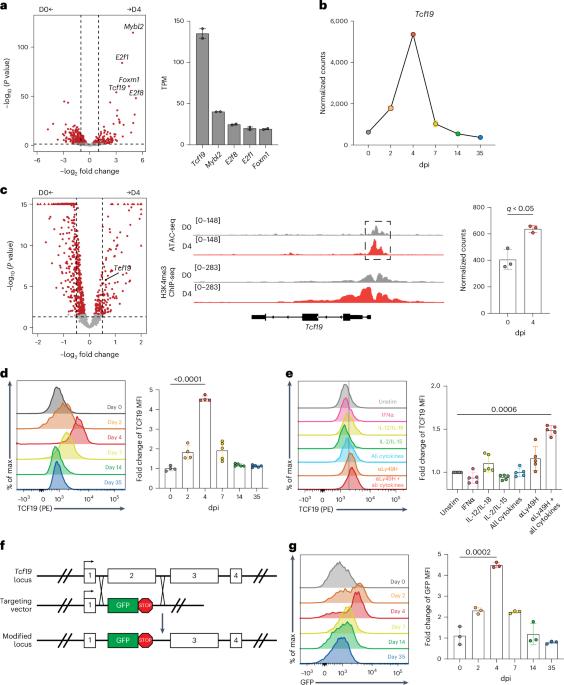
Natural killer (NK) cells are innate lymphocytes that play a critical role in host defense against viral infection. In addition to rapid effector cytokine production and direct cytotoxicity, NK cells exhibit features of adaptive immunity, including the capacity to undergo robust antigen-specific clonal proliferation and to generate immunological memory1–3. However, the transcriptional programs and regulators governing dynamic NK cell responses to viral infection have not been fully uncovered. In this study, we identified transcription factor 19 (TCF19) as a key driver of NK cell proliferation and calcium signaling in the context of mouse cytomegalovirus infection. Ablation of TCF19 was detrimental to NK cell clonal expansion and host protection against viral infection. Tcf19−/− NK cells were also unable to properly mobilize calcium downstream of antigen signaling to mediate cytotoxicity. Altogether, we find that TCF19 drives a transcriptional program that coordinates the innate and adaptive NK cell responses against viral infection. Sun and colleagues report that the transcription factor TCF19 regulates calcium signaling and cell cycling progression in NK cells and is required for innate and adaptive NK cell responses to viral infection.
TCF19驱动广泛的转录程序,增强抗病毒NK细胞的最佳先天和适应性功能
自然杀伤细胞(NK)是先天淋巴细胞,在宿主防御病毒感染中起关键作用。除了快速产生效应细胞因子和直接的细胞毒性外,NK细胞还表现出适应性免疫的特征,包括经历强大的抗原特异性克隆增殖和产生免疫记忆的能力1,2,3。然而,调控动态NK细胞对病毒感染反应的转录程序和调控因子尚未完全揭示。在这项研究中,我们发现转录因子19 (TCF19)是小鼠巨细胞病毒感染背景下NK细胞增殖和钙信号传导的关键驱动因素。TCF19的消融对NK细胞克隆扩增和宿主对病毒感染的保护作用不利。Tcf19−/−NK细胞也不能正确调动抗原信号下游的钙来介导细胞毒性。总之,我们发现TCF19驱动一个转录程序,协调先天和适应性NK细胞对病毒感染的反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


