Diet-derived galactose reprograms hepatocytes to prevent T cell exhaustion and elicit antitumour immunity
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
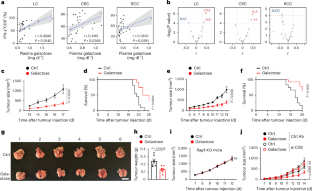
Dietary nutrients are inextricably linked to antitumour immune responses. However, the effect of diet-derived galactose on antitumour immunity remains unclear. Here we show that dietary galactose augments CD8+ T cell immunity to suppress tumour progression. High-galactose feeding drives hepatocyte-derived insulin-like growth factor binding protein 1 (IGFBP-1) production, thus restraining IGF-1 signalling-dependent T cell exhaustion. IGF-1 receptor (IGF-1R) deficiency in T cells potentiates antitumour CD8+ T cell responses and phenocopies high-galactose feeding by preventing T cell exhaustion. Circulating galactose reprograms hepatocyte metabolism to inactivate mTORC1, thereby inducing the production of IGFBP-1 to boost CD8+ T cell function. Furthermore, patients with cancer who have high plasma IGFBP-1 levels exhibit blocked T cell exhaustion and enhanced T cell responses in tumour tissues. These findings reveal that dietary galactose specifically elicits potent antitumour CD8+ T cell responses by facilitating hepatocyte-derived IGFBP-1 production, providing insights into the development of more effective immunotherapies against cancers. X. Du, W. Li, G. Li, C. Guo et al. report that dietary galactose regulates the cellular crosstalk between hepatocytes and CD8+ T cells via IGFBP-1 production and IGF-1 signalling, thereby modulating T cell exhaustion and antitumour immunity.

饮食来源的半乳糖重编程肝细胞,以防止T细胞衰竭和引发抗肿瘤免疫
膳食营养素与抗肿瘤免疫反应有着千丝万缕的联系。然而,饮食来源的半乳糖对抗肿瘤免疫的影响尚不清楚。本研究表明,饮食中的半乳糖可增强CD8+ T细胞免疫力,从而抑制肿瘤进展。高半乳糖喂养驱动肝细胞源性胰岛素样生长因子结合蛋白1 (IGFBP-1)的产生,从而抑制IGF-1信号依赖性T细胞衰竭。T细胞中IGF-1受体(IGF-1R)的缺乏增强了抗肿瘤CD8+ T细胞的反应,并通过防止T细胞衰竭来表型高半乳糖摄食。循环半乳糖重编程肝细胞代谢,使mTORC1失活,从而诱导IGFBP-1的产生,从而增强CD8+ T细胞的功能。此外,具有高血浆IGFBP-1水平的癌症患者表现出T细胞衰竭阻断和肿瘤组织中T细胞反应增强。这些发现表明,饮食中的半乳糖通过促进肝细胞来源的IGFBP-1的产生,特异性地引发了有效的抗肿瘤CD8+ T细胞反应,为开发更有效的癌症免疫疗法提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


