Unmet needs and emerging pharmacotherapies for autoimmune connective tissue disease-associated interstitial lung diseases
IF 8.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Autoimmune connective tissue disease associated with interstitial lung disease (CTD-ILD) includes rheumatoid arthritis-associated ILD, systemic sclerosis-associated-ILD, and several other ILDs. Many patients with CTD-ILD—as well as individuals with other ILDs—develop a progressive pulmonary fibrosis (PPF) similar to idiopathic pulmonary fibrosis (IPF). PPF is characterized by worsening respiratory symptoms, declining lung function despite current pharmacotherapies, and ultimately early death. Current pharmacotherapies for CTD-ILD and PPF include glucocorticoids, immunosuppressants, and anti-fibrotic agents. Due to the scarcity of randomized clinical trials for CTD-ILD, many pharmacotherapies are generally administered off-label (although several are approved in Japan), with notable exceptions including nintedanib, an anti-fibrotic agent approved for SSc-ILD and chronic progressive fibrosing ILD in several countries. As the available agents only slow the decline of pulmonary function and are associated with treatment-limiting side effects, there is a need for more efficacious and tolerable pharmacotherapies for CTD-ILD and PPF. Promising compounds in clinical trials include nerandomilast (a preferential phosphodiesterase 4B inhibitor), admilparant (a lysophosphatidic acid receptor 1 antagonist), and inhaled treprostinil (a prostacyclin analogue). Nerandomilast may have both anti-fibrotic and immunomodulatory properties; in preclinical models of PPF, it reduced neutrophils and macrophages and down-regulated pro-fibrotic signaling pathways. Hopefully, therefore, this pipeline will produce new medications to ease the collectively large burden of CTD-ILD and PPF.
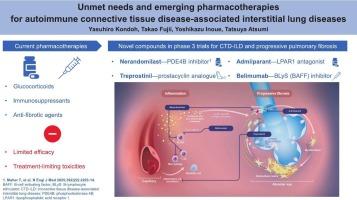
自身免疫性结缔组织病相关间质性肺疾病的未满足需求和新兴药物疗法
与间质性肺疾病相关的自身免疫性结缔组织疾病(CTD-ILD)包括类风湿关节炎相关的间质性肺疾病、系统性硬化症相关的间质性肺疾病和其他一些间质性肺疾病。许多患有ctd - ild的患者以及患有其他ild的个体发展为类似特发性肺纤维化(IPF)的进行性肺纤维化(PPF)。PPF的特点是呼吸系统症状恶化,尽管目前的药物治疗,肺功能下降,最终早期死亡。目前治疗CTD-ILD和PPF的药物包括糖皮质激素、免疫抑制剂和抗纤维化药物。由于缺乏CTD-ILD的随机临床试验,许多药物治疗通常是在标签外给药(尽管有几种在日本被批准),值得注意的例外包括nintedanib,一种抗纤维化药物,在几个国家被批准用于SSc-ILD和慢性进行性纤维化ILD。由于现有的药物只能减缓肺功能的下降,并伴有限制治疗的副作用,因此需要更有效和可耐受的药物治疗CTD-ILD和PPF。在临床试验中有前景的化合物包括nerandomilast(一种优先的磷酸二酯酶4B抑制剂)、admilparant(溶血磷脂酸受体1拮抗剂)和吸入性treprostiil(前列环素类似物)。尼兰多司特可能同时具有抗纤维化和免疫调节特性;在临床前PPF模型中,它减少了中性粒细胞和巨噬细胞,下调了促纤维化信号通路。因此,有希望的是,这一管道将产生新的药物,以减轻CTD-ILD和PPF的巨大负担。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Autoimmunity reviews
医学-免疫学
CiteScore
24.70
自引率
4.40%
发文量
164
审稿时长
21 days
期刊介绍:
Autoimmunity Reviews is a publication that features up-to-date, structured reviews on various topics in the field of autoimmunity. These reviews are written by renowned experts and include demonstrative illustrations and tables. Each article will have a clear "take-home" message for readers.
The selection of articles is primarily done by the Editors-in-Chief, based on recommendations from the international Editorial Board. The topics covered in the articles span all areas of autoimmunology, aiming to bridge the gap between basic and clinical sciences.
In terms of content, the contributions in basic sciences delve into the pathophysiology and mechanisms of autoimmune disorders, as well as genomics and proteomics. On the other hand, clinical contributions focus on diseases related to autoimmunity, novel therapies, and clinical associations.
Autoimmunity Reviews is internationally recognized, and its articles are indexed and abstracted in prestigious databases such as PubMed/Medline, Science Citation Index Expanded, Biosciences Information Services, and Chemical Abstracts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


