A complete model of mouse embryogenesis through organogenesis enabled by chemically induced embryo founder cells
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Embryo models offer opportunities for understanding development and advancing medicine but rely on intricate procedures with limitations in efficiency and developmental fidelity. Here, we employ a small-molecule-only approach to induce mouse embryonic stem cells into 8- to 16-cell-like embryo founder cells, enabling the generation of a complete embryo model. These founder cells specify all blastocyst lineages, both embryonic and extraembryonic, in vivo and in vitro. The embryo model made only from embryo founder cells faithfully recapitulates development through organogenesis. During gastrulation, it forms a primitive streak via epithelial-to-mesenchymal transition, generates the three germ layers, and develops an ectoplacental cone. The model proceeds to form 6–14 somite pairs, fore-/mid-/hindbrain, a looping heart tube, optic buds, allantois, tail bud, migrating primordial germ cells, and well-defined gut. Altogether, our system using embryo founder cells enables a direct, rapid, efficient, and accurate in vitro model of embryogenesis.
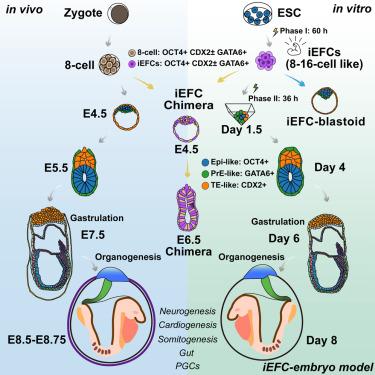
通过化学诱导胚胎建立细胞实现器官发生的小鼠胚胎发生的完整模型
胚胎模型为理解发育和推进医学提供了机会,但依赖于复杂的程序,在效率和发育保真度方面存在局限性。在这里,我们采用仅小分子的方法将小鼠胚胎干细胞诱导成8- 16个细胞样的胚胎建立细胞,从而能够生成完整的胚胎模型。这些创始细胞指定所有囊胚谱系,包括胚胎和胚胎外,体内和体外。仅由胚胎创始细胞制成的胚胎模型忠实地再现了器官发生的发育过程。在原肠胚形成过程中,原肠胚通过上皮细胞向间充质细胞的转变形成一条原始条纹,产生三个胚层,并发育出胎盘外锥体。模型继续形成6-14对体、前/中/后脑、一个环心管、视芽、尿囊、尾芽、迁移的原始生殖细胞和明确的肠道。总之,我们使用胚胎建立细胞的系统能够直接、快速、有效和准确地在体外建立胚胎发生模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


