Microwave-Assisted Methanol–Sulfosalicylic Acid Leaching System for Efficient and Closed-Loop Lithium-Ion Battery Cathode Recycling
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Effective recycling of spent lithium-ion batteries (LIBs) is critical to mitigating resource scarcity and environmental degradation amid rising global demand for energy storage. However, LIB recycling faces two persistent challenges: non-recyclable reductants in organic acid systems and inefficiencies in processing mixed cathode powders. Herein, we introduce a closed-loop methanol–sulfosalicylic acid (MeOH–SSA) system for rapid, sustainable metal recovery. Leveraging microwave-assisted leaching, this approach achieves exceptional efficiencies (>99% within 15 min) for extracting Li, Ni, Co, and Mn, governed by an internal diffusion-controlled mechanism with notably low activation energies (15.39, 17.57, and 17.55 kJ mol−1 for Ni, Co, and Mn, respectively). Our integrated recovery process, encompassing oxalate coprecipitation, MeOH regeneration, and Li3PO4 isolation, achieves complete recovery of Ni and Co, high recovery of Mn (97%), and effective Li recovery (95.87% with 94% purity), alongside 92.53% MeOH reuse. A techno-economic analysis highlights significant advantages: a net profit of $22.59 per kg of processed cathode material with an energy consumption of 28.09 MJ kg−1, outperforming conventional methods in cost-efficiency and environmental footprint. Notably, this system excels across both single-component and mixed cathode compositions. By simultaneously addressing reductant reusability and mixed-cathode compatibility, this work establishes a versatile, eco-efficient framework for LIB recycling.
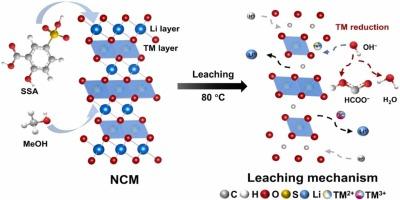
微波辅助甲醇-磺基水杨酸浸出系统高效闭环锂离子电池正极回收
在全球能源存储需求不断增长的背景下,有效回收废旧锂离子电池对于缓解资源短缺和环境恶化至关重要。然而,锂离子电池的回收面临两个持续的挑战:有机酸体系中不可回收的还原剂和混合阴极粉末加工的低效率。在此,我们介绍了一个闭环甲醇-磺基水杨酸(MeOH-SSA)系统,用于快速,可持续的金属回收。利用微波辅助浸出,该方法在提取Li, Ni, Co和Mn方面获得了卓越的效率(15分钟内达到99%),由内部扩散控制机制控制,活化能明显较低(Ni, Co和Mn分别为15.39,17.57和17.55 kJ mol−1)。我们的综合回收工艺包括草酸共沉淀、MeOH再生和Li3PO4分离,实现了Ni和Co的完全回收,Mn的高回收率(97%),Li的有效回收率(95.87%,纯度为94%),以及92.53%的MeOH回用。技术经济分析强调了显著的优势:每公斤加工阴极材料的净利润为22.59美元,能耗为28.09 MJ kg - 1,在成本效益和环境足迹方面优于传统方法。值得注意的是,该系统在单组分和混合阴极成分方面都表现出色。通过同时解决还原剂的可重用性和混合阴极的兼容性,这项工作建立了一个多功能的、生态高效的LIB回收框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


