Mechanochemical coupling of cell shape and organ function optimizes heart size and contractile efficiency in zebrafish
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
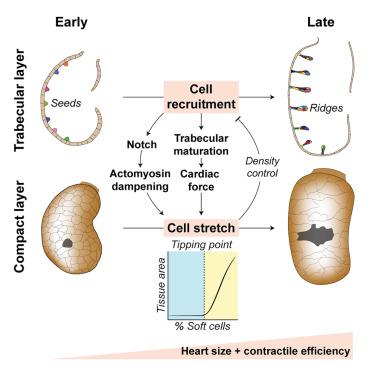
How simple tissue primordia sculpt complex functional organs, robustly and reproducibly, remains elusive. During zebrafish development, the embryonic myocardial wall matures into an intricate 3D architecture, composed of an outer compact layer enveloping an inner layer of multicellular trabecular ridges. How these tissue layers acquire their characteristic form suited for their function remains an open question. Here, we find that multiscale mechanochemical coupling and an emergent tissue-scale morphological transition steer functional maturation of the developing zebrafish heart. Single-celled trabecular seeds recruit outer compact layer cells to mature into clonally heterogeneous multicellular ridges, thereby amplifying cardiac contractile forces. In response, the remaining compact layer cells are stretched, which impedes their further recruitment, thereby constraining trabecular ridge density. Concomitantly, Notch-dependent actomyosin dampening triggers a sharp transition in myocardial tissue area, activating rapid organ growth that expands blood-filling capacity. Thus, multiscale self-organizing interactions optimize heart size and contractile efficiency to support embryonic life.
细胞形状和器官功能的机械化学耦合优化了斑马鱼的心脏大小和收缩效率
如何简单的组织原基雕刻复杂的功能器官,稳健和可复制,仍然是难以捉摸的。在斑马鱼发育过程中,胚胎心肌壁成熟为复杂的三维结构,由外层致密层包裹着内层多细胞小梁脊组成。这些组织层如何获得适合其功能的特征形式仍然是一个悬而未决的问题。在这里,我们发现多尺度的机械化学耦合和一个新兴的组织尺度的形态转变引导发育中的斑马鱼心脏的功能成熟。单细胞小梁种子吸收外层致密层细胞成熟为克隆异质多细胞脊,从而增强心脏收缩力。作为回应,剩余的致密层细胞被拉伸,这阻碍了它们的进一步募集,从而限制了小梁脊的密度。同时,缺口依赖的肌动球蛋白抑制触发心肌组织面积的急剧转变,激活器官的快速生长,扩大充血能力。因此,多尺度自组织相互作用优化心脏大小和收缩效率,以支持胚胎生命。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


