Facet-selective zinc deposition and kinetics enhancement via aromatic and zinc-affinitive compounds for long-term stable zinc anodes
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Aqueous zinc-ion batteries (AZIBs) face critical challenges due to zinc dendrite growth and hydrogen evolution reactions, which degrade both battery performance and longevity. To address these issues, we introduce phenethyl alcohol (PA), a multifunctional additive that enhances zinc anode stability by regulating the solvation structure of Zn2+ ions and promoting facet-selective zinc deposition. The hydroxyl group of PA exhibits a strong affinity for zinc ions, reconfiguring the solvation shell by displacing coordinated water molecules, which effectively suppresses HER. Additionally, the conjugated aromatic π-π stacking interactions between PA's benzene ring and the zinc (002) crystal facet direct the preferential deposition of zinc on this low-energy plane, significantly improving deposition kinetics and ensuring a uniform zinc layer. This dual mechanism not only stabilizes the zinc interface but also enhances the cycling reversibility of the anode. As a result, symmetric cells with PA-modified electrolytes achieve over 1600 h of stable cycling at 2 mA cm−2, while Zn||Ti half-cells maintain a remarkable Coulombic efficiency of 99.74 % at 4 mA cm−2. These findings underscore PA's potential as a highly effective electrolyte additive, offering a promising strategy for improving both the electrochemical performance and long-term stability of zinc anodes in the next generation of high-performance AZIBs.
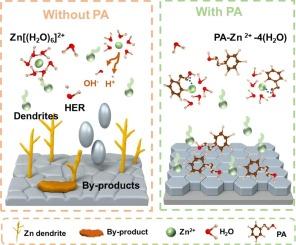
芳香族和亲锌化合物对长期稳定锌阳极表面选择性锌沉积和动力学增强的影响
由于锌枝晶生长和析氢反应,水性锌离子电池(azib)面临着严峻的挑战,这将降低电池的性能和寿命。为了解决这些问题,我们引入了苯乙醇(PA),这是一种多功能添加剂,通过调节Zn2+离子的溶剂化结构和促进表面选择性锌沉积来提高锌阳极的稳定性。PA的羟基对锌离子表现出很强的亲和力,通过取代配位水分子重新配置溶剂化壳,有效抑制HER。此外,PA的苯环与锌(002)晶面之间的共轭芳香π-π堆叠相互作用直接导致锌在低能平面上优先沉积,显著改善沉积动力学并确保锌层均匀。这种双重机制不仅稳定了锌界面,而且提高了阳极的循环可逆性。结果表明,具有pa修饰电解质的对称电池在2 mA cm−2下实现了超过1600 h的稳定循环,而Zn||Ti半电池在4 mA cm−2下保持了99.74 %的显着库仑效率。这些发现强调了PA作为一种高效电解质添加剂的潜力,为提高下一代高性能azib锌阳极的电化学性能和长期稳定性提供了有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


