Establishment of human gastrulating stem cells with the capacity of stable differentiation into multiple gastrulating cell types
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Pluripotent stem cells (PSCs) have been derived from various species, but most culture systems stabilize only a single PSC type. By contrast, epiblast cells in vivo exist along a continuum and interact dynamically with both embryonic and extraembryonic cells, interactions missing in standard PSC cultures. This absence limits the self-organizing potential of PSCs and leads to disorganized tissue formation in teratomas. To address this, we developed a unified culture system that supports the stable differentiation of epiblast-like cells into multiple key human gastrulating cell types, collectively called human gastrulating stem cells (hGaSCs). hGaSCs, composed of endoderm-like, mesoderm-like, ectoderm-like, amnion ectoderm-like, and primordial germ cell-like cells, maintain a stable balance during long-term culture. In 3D culture, hGaSCs self-assemble into gastruloid-like structures (hGaSC-gastruloids) that model aspects of a Carnegie Stage 7 human embryo, including gastrulation and germ layer specification. Using hGaSC-gastruloids, we modeled the effects of valproic acid (VPA) on human gastrulation and uncovered molecular pathways underlying VPA-induced malformations. When transplanted into the seminiferous tubules, hGaSCs formed embryo-like structures, progressing through fetal tissue and organ development, unlike the disorganized growth seen in teratomas. In conclusion, hGaSCs provide a versatile platform to study human gastrulation, early organogenesis, developmental defects, and drug teratogenicity, with promising applications in tissue and organ generation from cultured stem cells.

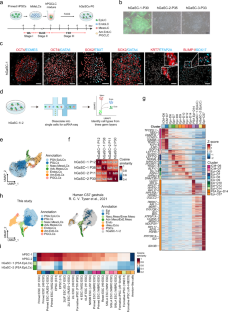
建立具有稳定分化为多种原肠胚细胞类型的人原肠胚干细胞
多能干细胞(PSCs)来源于多种物种,但大多数培养系统只能稳定一种类型的多能干细胞。相比之下,外胚层细胞在体内是连续存在的,并与胚胎细胞和胚胎外细胞动态地相互作用,这种相互作用在标准的PSC培养中是缺失的。这种缺失限制了PSCs的自组织潜能,导致畸胎瘤中组织形成混乱。为了解决这个问题,我们开发了一个统一的培养系统,该系统支持上皮样细胞稳定地分化为多种关键的人类原肠形成细胞类型,统称为人类原肠形成干细胞(hGaSCs)。hGaSCs由内胚层样细胞、中胚层样细胞、外胚层样细胞、羊膜外胚层样细胞和原始生殖细胞样细胞组成,在长期培养过程中保持稳定的平衡。在3D培养中,hgasc自组装成类胃原体结构(hgasc -类胃原体),模拟卡内基7期人类胚胎的各个方面,包括原肠形成和胚层规范。利用hgasc -类胃原液,我们模拟了丙戊酸(VPA)对人原肠胚形成的影响,并揭示了VPA诱导的畸形的分子途径。当移植到精小管中时,hGaSCs形成胚胎样结构,通过胎儿组织和器官发育进展,这与畸胎瘤中看到的无序生长不同。总之,hGaSCs为研究人类原肠胚形成、早期器官发生、发育缺陷和药物致畸提供了一个多功能平台,在培养干细胞的组织和器官生成方面具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


