The role of redox homeostasis in tumor progression: implications for cancer therapy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The dynamic balance of reduction–oxidation (redox) plays a vital role in maintaining normal physiological functions, such as metabolism, cell differentiation, immune response, and cell death. The disruption of redox homeostasis in tumor cells leads to more adverse damage than that in normal tissues because of the significantly higher redox level resulting from the high metabolic characteristics of tumor tissue. However, tremendous efforts based on the regulation of redox homeostasis are still hampered by the enhanced antioxidant capability of tumor cells during treatment. Hence, an in-depth introduction regarding the relationship between tumors and oxidative stress, involving the factors leading to oxidative stress and the impact of oxidative stress on tumor progression, is urgently required for the development of cancer therapy strategies with robust antitumor effects. Thus, we systematically introduced the relationship between tumors and oxidative stress. We have also included another section to introduce the recent successes in the trial of oxidative stress-induced strategies based on nanomedicine.
Statement of significance
Since cancer cells exhibit a unique, enhanced dynamic maintenance of redox homeostasis, this review is composed from a fresh perspective of the paradoxical crosstalk between redox homeostasis and tumor progression. We propose the disruption of redox balance acts as an intricate inducer for various forms of programmed cell death (PCD), including apoptosis, pyroptosis, ferroptosis, autophagy, cuproptosis, and disulfidptosis. Diverse strategies capable of amplifying oxidative stress for potent cancer treatment are subsequently summarized. The existing challenges together with the mitigation solutions are finally discussed. This review highlights the significant role of redox homeostasis in tumor progression, and is believed to bridge the gap between fundamental researches and clinical translations of nanomedicines developed in this research field.
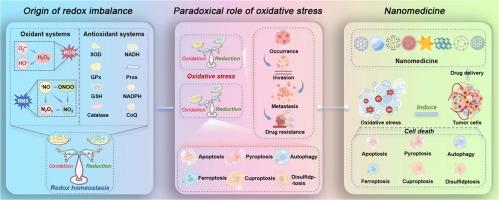
氧化还原稳态在肿瘤进展中的作用:对癌症治疗的意义。
还原-氧化(redox)的动态平衡在维持正常的生理功能(如代谢、细胞分化、免疫反应和细胞死亡)中起着至关重要的作用。由于肿瘤组织的高代谢特性导致氧化还原水平显著升高,因此肿瘤细胞中氧化还原稳态的破坏导致比正常组织更严重的不良损伤。然而,基于氧化还原稳态调节的巨大努力仍然受到肿瘤细胞在治疗过程中抗氧化能力增强的阻碍。因此,深入了解肿瘤与氧化应激的关系,包括导致氧化应激的因素以及氧化应激对肿瘤进展的影响,是开发具有强大抗肿瘤作用的癌症治疗策略的迫切需要。因此,我们系统地介绍了肿瘤与氧化应激之间的关系。我们还包括另一个部分,介绍了最近在氧化应激诱导策略的纳米医学试验中取得的成功。意义声明:由于癌细胞表现出独特的、增强的氧化还原稳态动态维持,本综述从氧化还原稳态和肿瘤进展之间矛盾的串扰的新视角组成。我们提出氧化还原平衡的破坏是多种形式的程序性细胞死亡(PCD)的复杂诱导剂,包括细胞凋亡、焦亡、铁亡、自噬、铜亡和二硫亡。随后总结了各种能够放大氧化应激的有效癌症治疗策略。最后讨论了现有的挑战以及缓解挑战的解决办法。这篇综述强调了氧化还原稳态在肿瘤进展中的重要作用,并被认为是该研究领域纳米药物的基础研究和临床应用之间的桥梁。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


