Rational engineering of allosteric protein switches by in silico prediction of domain insertion sites
IF 32.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Domain insertion engineering is a powerful approach to juxtapose otherwise separate biological functions, resulting in proteins with new-to-nature activities. A prominent example are switchable protein variants, created by receptor domain insertion into effector proteins. Identifying suitable, allosteric sites for domain insertion, however, typically requires extensive screening and optimization. We present ProDomino, a machine learning pipeline to rationalize domain recombination, trained on a semisynthetic protein sequence dataset derived from naturally occurring intradomain insertion events. ProDomino robustly identifies domain insertion sites in proteins of biotechnological relevance, which we experimentally validated in Escherichia coli and human cells. Finally, we used light- and chemically regulated receptor domains as inserts and demonstrate the rapid, model-guided creation of potent, single-component opto- and chemogenetic protein switches. These include novel CRISPR–Cas9 and –Cas12a variants for inducible genome engineering in human cells. Our work enables one-shot domain insertion engineering and substantially accelerates the design of customized allosteric proteins. ProDomino is a machine leaning-based method, trained on a semisynthetic domain insertion dataset, to guide the engineering of protein domain recombination.
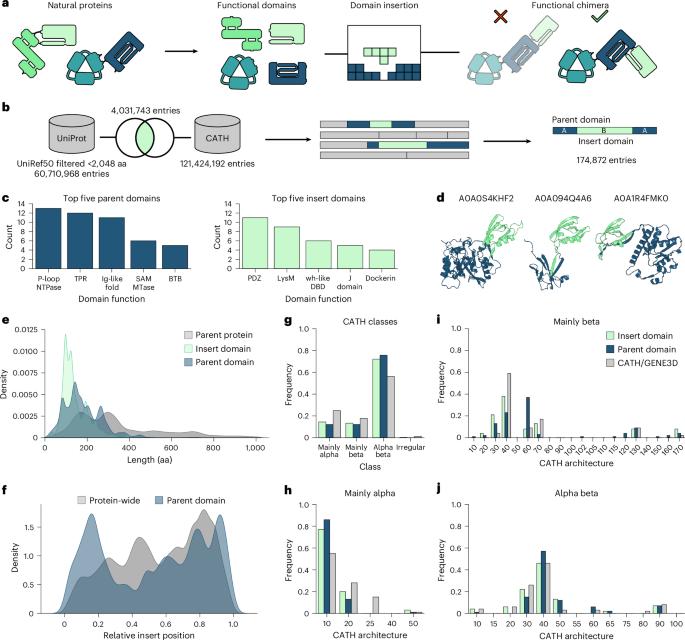
通过计算机预测结构域插入位点,实现变构蛋白开关的合理工程。
结构域插入工程是一种强大的方法,可以将不同的生物功能并置,从而产生具有新自然活性的蛋白质。一个突出的例子是可切换的蛋白质变体,通过受体结构域插入到效应蛋白中产生。然而,确定合适的变构位点进行结构域插入通常需要广泛的筛选和优化。我们提出了ProDomino,这是一个机器学习管道,用于合理化结构域重组,该管道在来自自然发生的结构域内插入事件的半合成蛋白质序列数据集上进行训练。ProDomino强有力地识别了与生物技术相关的蛋白质中的结构域插入位点,我们在大肠杆菌和人类细胞中实验验证了这一点。最后,我们使用光和化学调节受体结构域作为插入,并展示了快速,模型引导的高效,单组分光和化学发生蛋白开关的创建。其中包括用于人类细胞诱导基因组工程的新型CRISPR-Cas9和-Cas12a变体。我们的工作实现了一次性结构域插入工程,并大大加快了定制化变构蛋白的设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Methods
生物-生化研究方法
CiteScore
58.70
自引率
1.70%
发文量
326
审稿时长
1 months
期刊介绍:
Nature Methods is a monthly journal that focuses on publishing innovative methods and substantial enhancements to fundamental life sciences research techniques. Geared towards a diverse, interdisciplinary readership of researchers in academia and industry engaged in laboratory work, the journal offers new tools for research and emphasizes the immediate practical significance of the featured work. It publishes primary research papers and reviews recent technical and methodological advancements, with a particular interest in primary methods papers relevant to the biological and biomedical sciences. This includes methods rooted in chemistry with practical applications for studying biological problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


