Impact mechanisms of humic acid on the behavior of CMC-stabilized nZVI in groundwater: Implications for long-term chlorinated contaminant remediation
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Stabilized nanoscale zero valent iron (nZVI) is commonly used as a reductant during groundwater remediation of halogenated hydrocarbons. However, humic acid (HA) in groundwater can affect the environmental behavior of nZVI during contaminant degradation. This study investigated the effects of HA on carboxymethyl cellulose-stabilized nZVI (CMC-nZVI) by studying hydrogen evolution, Fe0 corrosion and carbon tetrachloride (CT) degradation kinetics. The results show that, during the early stage (≤ 24 h), low-concentration HA (HA/CMC-nZVI: 2–8) enhanced the hydrogen evolution reaction (HER) of CMC-nZVI up to 1.36 fold by forming Fe2+-HA complexes that prevented passivation. In contrast, high-concentration HA (mass ratio: 24) inhibited HER during the entire reaction period, as HA rapidly and directly consumed Fe0 from the start of the reaction (final Fe0 consumption by HA: 62.0 %). In this study we also developed a modified differential HER approach to quantitatively determine HA-induced Fe0 consumption. This confirmed that quinone moieties in HA compete for electrons and are reduced to hydroquinones, thereby consuming Fe0. In CT degradation, HA accelerated the initial reaction (rate constant kCT increased from 0.010 min−1 to 0.038 min−1) but significantly reduced electron efficiency (from 6.4 % to 1.3 %) and material longevity at HA/CMC-nZVI ratio of 2–24. Due to HA-mediated depletion of CMC-nZVI, the residual Fe0 had limited capacity for CT degradation after 24 h. The column experiments further confirmed this adverse effect of HA. However, the coexisting ions in actual groundwater were found to mitigate the Fe0 consumption induced by HA. This study reveals HA's dual role in CT remediation: initially HA enhances ZVI reactivity, but subsequently HA reduces ZVI reactivity due to electron competition. This provides novel insights for the optimization of ZVI dosing in groundwater remediation.
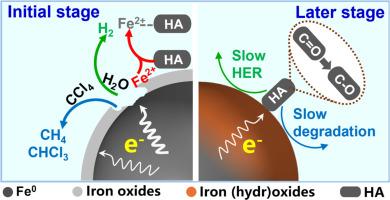
腐植酸对地下水中cmc稳定的nZVI行为的影响机制:对长期氯化污染物修复的影响
稳定纳米级零价铁(nZVI)是地下水卤化烃修复中常用的还原剂。然而,地下水中的腐植酸(HA)会影响nZVI在污染物降解过程中的环境行为。本研究通过研究HA对羧甲基纤维素稳定nZVI (CMC-nZVI)的析氢、Fe0腐蚀和四氯化碳(CT)降解动力学来研究HA对CMC-nZVI的影响。结果表明,在早期(≤24 h),低浓度HA (HA/CMC-nZVI: 2-8)通过形成阻止钝化的Fe2+-HA配合物,使CMC-nZVI的析氢反应(HER)增强了1.36倍。相反,高浓度的HA(质量比为24)在整个反应期间抑制HER,因为HA从反应开始就迅速直接消耗Fe0(最终HA消耗Fe0: 62.0%)。在这项研究中,我们还开发了一种改进的差分HER方法来定量测定ha诱导的Fe0消耗。这证实了HA中的醌部分竞争电子并被还原为对苯二酚,从而消耗Fe0。在CT降解中,HA加速了初始反应(速率常数kCT从0.010 min−1增加到0.038 min−1),但在HA/CMC-nZVI比为2-24时,显著降低了电子效率(从6.4%降至1.3%)和材料寿命。由于HA介导CMC-nZVI的损耗,残留的Fe0在24 h后对CT的降解能力有限。柱实验进一步证实了HA的这一不利影响。而实际地下水中共存离子的存在对HA诱导的Fe0消耗有一定的抑制作用。本研究揭示了HA在CT修复中的双重作用:最初HA增强了ZVI的反应性,但随后由于电子竞争,HA降低了ZVI的反应性。这为地下水修复中ZVI剂量的优化提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


