A green approach for electromembrane extraction of morphine from urine using sodium alginate-g-polyacrylic acid/agarose hydrogel membrane
IF 6.5
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
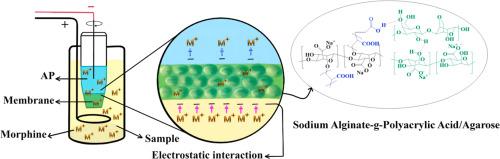
The development of novel membranes through green chemistry remains a significant challenge in advancing practical separation science. In this study, a sodium alginate-g-polyacrylic acid/agarose hydrogel was synthesized and, for the first time, applied as a membrane in gel electromembrane extraction (G-EME) for the isolation of morphine, a basic analyte, from urine samples. The extracted morphine was subsequently quantified using differential pulse voltammetry (DPV) with a glassy carbon electrode. The hydrogel membrane (5 mm thick) was prepared using 0.75 % (w/v) sodium alginate, 3.80 % (v/v) acrylic acid, 0.04 % (w/v) ammonium persulfate, and 1.25 % (w/v) agarose. The extraction process was optimized by evaluating factors such as membrane composition, extraction time, applied voltage, and pHs of the sample solution, membrane, and acceptor phase (AP). Under optimal conditions (25 min extraction time, 70 V applied voltage, membrane pH 4.0, AP pH 3.0, and sample solution pH 7.0) morphine was efficiently extracted through the hydrogel membrane and transferred to an aqueous AP. The collected AP was mixed with 0.15 M phosphate buffer (pH 7.0) and analyzed voltammetrically. The method achieved a limit of detection of 0.09 μg/mL and a limit of quantification of 0.28 μg/mL, and was successfully applied for morphine determination in urine sample. The polyacrylic acid-based membrane enhanced extraction efficiency through electrostatic interactions, while the use of DPV offered clearer signal interpretation and reduced background current, facilitating reliable detection of trace analytes. These advantages support the method’s potential for simple, selective, and environmentally friendly analysis of basic drugs in biological samples.
海藻酸钠-聚丙烯酸/琼脂糖水凝胶膜电膜提取尿液中吗啡的绿色方法
通过绿色化学开发新型膜仍然是推进实际分离科学的重大挑战。本研究合成了海藻酸钠-g-聚丙烯酸/琼脂糖水凝胶,并首次应用于凝胶电膜萃取(G-EME)分离尿液中碱性分析物吗啡。随后用玻璃碳电极用差分脉冲伏安法(DPV)对提取的吗啡进行定量。以0.75% (w/v)的海藻酸钠、3.80% (v/v)的丙烯酸、0.04% (w/v)的过硫酸铵和1.25% (w/v)的琼脂糖为原料制备了厚度为5mm的水凝胶膜。通过考察膜组成、提取时间、外加电压、样品溶液ph值、膜和受体相(AP)等因素对提取工艺进行优化。在最佳条件下(提取时间25 min,施加电压70 V,膜pH 4.0, AP pH 3.0,样品溶液pH 7.0),通过水凝胶膜有效提取吗啡,并将其转移到水溶液中。将收集的AP与0.15 M磷酸盐缓冲液(pH 7.0)混合,并进行伏安分析。该方法的检出限为0.09 μg/mL,定量限为0.28 μg/mL,可成功用于尿样中吗啡的检测。聚丙烯酸基膜通过静电相互作用提高了提取效率,而DPV的使用提供了更清晰的信号解释,降低了背景电流,促进了痕量分析物的可靠检测。这些优点支持了该方法对生物样品中基本药物进行简单、选择性和环境友好分析的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


