Metabolic engineering reveals the relative importance of different sugar catabolic pathways during consumption of plant biomass by Aspergillus niger
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
Abstract
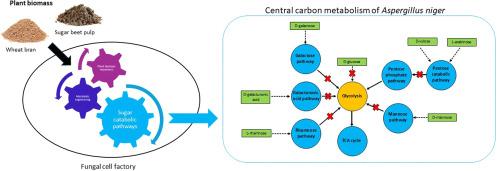
To evaluate the impact of individual sugar catabolic pathways on the physiology of A. niger when using plant biomass as a carbon source, key pathways converting plant biomass derived monomers were blocked. The resulting deletion mutants were analyzed using wheat bran and sugar beet pulp as substrates. On both substrates, the strongest affected single-pathway mutants were the pentose catabolic pathway (PCP) (ΔxkiA) and glycolysis (ΔhxkAΔglkA) deficient mutants. On wheat bran, which is rich in pentose sugars, blocking the PCP by deletion of xkiA strongly impacted both growth and gene expression. However, the effect was even stronger in ΔhxkAΔglkA and similar to a strain in which all pathways were blocked, highlighting the crucial role of glycolysis and/or carbon catabolite repression in A. niger physiology. These results demonstrate the complexity of A. niger metabolism during growth on plant biomass and provide insights into aspects to consider during metabolic engineering to obtain fungal cell factories.
代谢工程揭示了黑曲霉消耗植物生物量过程中不同糖分解代谢途径的相对重要性
为了评估以植物生物量为碳源时单个糖分解代谢途径对黑曲霉生理的影响,阻断了转化植物生物量衍生单体的关键途径。以麦麸和甜菜浆为底物对缺失突变体进行分析。在这两种底物上,受影响最大的单途径突变体是戊糖分解代谢途径(PCP) (ΔxkiA)和糖酵解(ΔhxkAΔglkA)缺陷突变体。在富含戊糖的麦麸中,通过缺失xkiA阻断PCP对生长和基因表达均有显著影响。然而,这种影响在ΔhxkAΔglkA中甚至更强,类似于所有途径被阻断的菌株,突出了糖酵解和/或碳分解代谢抑制在黑曲霉生理学中的关键作用。这些结果表明了黑曲霉在植物生物量生长过程中代谢的复杂性,并为获得真菌细胞工厂的代谢工程提供了一些参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


