A comprehensive study of CO2 capture using attapulgite and novel hybrid Attapulgite/13X zeolite composite: kinetic, isotherm, and thermodynamic analysis
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
In this study, a novel attapulgite/13X zeolite composite was synthesized in varying ratios (1:1, 2:1, 1:2) and evaluated for enhanced CO2 capture performance. The composite was prepared via a simple hydrothermal method and characterized through XRD, FTIR, BET, SEM-EDX, and TGA analyses. Results confirmed improved structural stability, increased surface area, and greater porosity relative to pristine attapulgite. The composite with a 1:2 ATP/Z13X(13X zeolite) ratio demonstrated the highest CO2 adsorption capacity (2.2 mmol g−1) at 25 °C, nearly tenfold higher than that of pure attapulgite (0.21 mmol g−1), owing to improved textural characteristics and synergistic effects between components. Adsorption was favored at lower temperatures and higher adsorbent dosages, while elevated CO2 partial pressures enhanced uptake capacity. Kinetic analyses indicated that physisorption governed the process, best described by the pseudo-first order and Elovich models. The adsorption mechanism conformed well to the Freundlich and Dubinin isotherms, consistent with multilayer sorption on heterogeneous surfaces. Thermodynamic evaluations revealed that the process is spontaneous and exothermic, with ΔG° ranging from −11.15 to −11.69 kJ mol−1 and ΔH° of −9.70 kJ mol−1, confirming the physical nature of adsorption. The composite also exhibited excellent cyclic stability over 11 regeneration cycles with only a 2.8 % capacity loss. These findings demonstrate the composite's promise as a cost-effective and durable adsorbent for post-combustion CO2 capture applications.
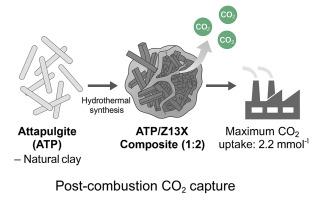
利用凹凸棒石和新型混合凹凸棒石/13X沸石复合材料进行CO2捕集的综合研究:动力学、等温线和热力学分析
在这项研究中,以不同的比例(1:1,2:1,1:2)合成了一种新型凹凸棒石/13X沸石复合材料,并对其增强的二氧化碳捕获性能进行了评估。采用简单水热法制备了该复合材料,并通过XRD、FTIR、BET、SEM-EDX和TGA分析对其进行了表征。结果证实,与原始凹凸棒石相比,结构稳定性更好,表面积增加,孔隙度更高。当ATP/Z13X(13X沸石)比为1:2时,复合材料在25°C时的CO2吸附量最高(2.2 mmol g−1),比纯凹凸棒土(0.21 mmol g−1)高出近10倍,这是由于其结构特性的改善和组分之间的协同作用。较低温度和较高吸附剂用量有利于吸附,而提高CO2分压可增强吸附能力。动力学分析表明,物理吸附控制了这一过程,最好的描述是伪一阶和Elovich模型。吸附机理符合Freundlich和Dubinin等温线,符合多相表面的多层吸附。热力学评价表明,该过程为自发放热过程,ΔG°范围为−11.15 ~−11.69 kJ mol−1,ΔH°范围为−9.70 kJ mol−1,证实了吸附的物理性质。该复合材料在11次再生循环中表现出良好的循环稳定性,容量损失仅为2.8%。这些发现表明,这种复合材料有望成为一种具有成本效益和耐用的吸附剂,用于燃烧后的二氧化碳捕获应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


