Gynecologic postoperative anti-adhesion barriers: From biomaterials to barrier development
Q3 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
Gynecologic postoperative adhesions (GPOA) remain an under-appreciated source of morbidity despite advances in minimally invasive surgery. Adhesions forming after myomectomy, extensive endometriosis excision, repeat caesarean section, or hysteroscopic adhesiolysis develop in 20 – 90 % of patients and account for up to 40 % of secondary infertility, chronic pelvic pain, bowel obstruction, and life-threatening obstetric complications such as placenta accreta spectrum. Because the uterus is hormonally responsive and destined for potential pregnancy, anti-adhesion barriers for gynecologic tissues must meet stricter criteria for biocompatibility, resorption timing, teratogenic safety, and reproductive regulatory classification than barriers designed for bowel or tendon repair.
This review consolidates the rapidly expanding literature on biomaterial-based barriers specifically tailored for gynecologic applications. We first dissect the pathophysiology of uterine and adnexal adhesion formation—including mesothelial disruption, fibrin persistence, and estrogen-modulated wound remodeling—to highlight design targets unique to the female reproductive tract. Next, we critically appraise natural and synthetic polymers, discussing how formulation parameters govern in-vivo elimination or excretion routes and influence fertility outcomes. Cutting-edge fabrication strategies—such as electrospinning, 3D bioprinting, melt electrowriting, Janus hydrogels, and microneedle patches—are reviewed with an eye toward uterine conformity, minimally invasive deployability, and on-demand release of drugs or exosomes. We further map current FDA-cleared films (INTERCEED™, Seprafilm®, SurgiWrap®) against unmet gynecologic needs, delineate limitations, and identify opportunities for multifunctional, self-healing, image-visible, and patient-specific barrier platforms.
By framing adhesion prevention through a gynecologic lens, this article provides clinicians, materials scientists, and device developers with a roadmap for translating next-generation barriers from bench to bedside, ultimately reducing infertility, surgical re-admission, and obstetric risk in women worldwide.
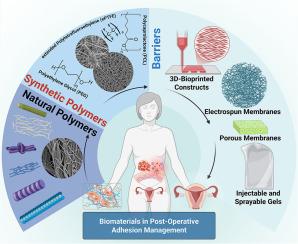
妇科术后抗粘连屏障:从生物材料到屏障的发展
尽管微创手术取得了进展,妇科术后粘连(GPOA)仍然是一个未被充分认识的发病率来源。20 - 90%的患者在子宫肌瘤切除术、广泛子宫内膜异位症切除术、重复剖宫产或宫腔镜下粘连松解术后形成粘连,占继发性不孕症、慢性盆腔疼痛、肠梗阻和危及生命的产科并发症(如胎盘增生)的40%。由于子宫对激素有反应,并且注定要怀孕,因此妇科组织的抗粘连屏障必须满足比用于肠或肌腱修复的屏障更严格的生物相容性、吸收时间、致畸安全性和生殖调节分类标准。这篇综述整合了快速扩展的专门针对妇科应用的生物材料屏障的文献。我们首先剖析子宫和附件粘连形成的病理生理学,包括间皮破坏、纤维蛋白持久性和雌激素调节的伤口重塑,以突出女性生殖道独特的设计目标。接下来,我们批判性地评估天然和合成聚合物,讨论配方参数如何控制体内消除或排泄途径并影响生育结果。尖端的制造策略-如静电纺丝,3D生物打印,熔融电书写,Janus水凝胶,微针贴片-回顾着眼于子宫整合,微创部署,按需释放药物或外泌体。我们进一步将目前fda批准的膜(INTERCEED™,separfilm®,SurgiWrap®)与未满足的妇科需求进行对比,描述局限性,并确定多功能,自我修复,图像可见和患者特异性屏障平台的机会。通过从妇科角度构建粘连预防,本文为临床医生、材料科学家和设备开发人员提供了将下一代屏障从实验台转化为床边的路线图,最终减少全世界妇女的不孕症、手术再入院和产科风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


