δ-MnO2 nanoplates@N-doped hollow carbon spheres for efficient uranium extraction via coordination-electrostatic synergy
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
To meet the pressing demands of sustainable nuclear energy development and radioactive pollution mitigation, the development of high-efficiency adsorbents for uranium extraction from real wastewater is paramount. In this study, a MNP@NHCS adsorbent was engineered through a carrier-supported strategy involving in-situ growth of δ-MnO2 nanoplates (MNP) on N-doped hollow carbon spheres (NHCS). Material characterization confirmed that NHCS suppresses δ-MnO2 agglomeration and enhances the specific surface area to 218.22 m2/g (3.5-fold higher than pristine δ-MnO2 62.28 m2/g). Batch experiments demonstrated exceptional performance: excellent U(VI) adsorption capacity (423.61 mg/g), outstanding selectivity (Kd = 1.9 × 105 mL/g), robust recyclability (>95 % removal over 8 adsorption-desorption cycles), and real wastewater breakthrough (95 % uranium recovery rate: 0.21 mg U(VI) / 100 mg ads.). Comprehensive characterization, multi-model fit analysis and in-situ zeta potential monitoring revealed that U(VI) capture follows a spontaneous, endothermic monolayer process driven by coordination-electrostatic synergy between Lewis acid-base sites (Mn-O, -OH, and -NH2) and electrostatic attraction. This work provides an efficient, stable, and eco-friendly adsorbent for real wastewater treatment in the nuclear industry, advancing uranium resource recovery and sustainable nuclear energy development.
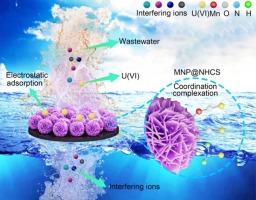
δ-MnO2 nanoplates@N-doped空心碳球配位-静电协同萃取铀
为了满足核能可持续发展和减轻放射性污染的迫切需求,开发高效的从实际废水中提取铀的吸附剂是至关重要的。在本研究中,通过载流子负载策略设计了MNP@NHCS吸附剂,该策略涉及在n掺杂空心碳球(NHCS)上原位生长δ-MnO2纳米板(MNP)。材料表征证实,NHCS抑制了δ-MnO2团聚,将比表面积提高到218.22 m2/g(比原始δ-MnO2 62.28 m2/g高3.5倍)。批量实验证明了优异的性能:出色的U(VI)吸附能力(423.61 mg/g),出色的选择性(Kd = 1.9 × 105 mL/g),强大的可回收性(8次吸附-解吸循环去除95%),以及真正的废水突破(95%铀回收率:0.21 mg U(VI) / 100 mg ads.)。综合表征、多模型拟合分析和原位zeta电位监测表明,U(VI)捕获遵循一个自发的吸热单层过程,由Lewis酸碱位点(Mn-O、-OH和-NH2)和静电吸引之间的配位-静电协同作用驱动。本研究为核工业实际废水处理提供了一种高效、稳定、环保的吸附剂,促进了铀资源的回收和核能的可持续发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


