Pseudomonas infections persisting after CFTR modulators are widespread throughout the lungs and drive lung inflammation
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators improve the physiological defect causing cystic fibrosis, but the lungs of most people remain infected and inflamed. A leading hypothesis implicates damaged segments as the cause of persistent infection and predicts that mildly diseased segments within an individual's lungs will clear after treatment, whereas severely diseased segments will not. Our findings contradict this hypothesis. We used bronchoscopy to sample the least- and most-damaged lung segments in Pseudomonas aeruginosa (Pa)-infected individuals before modulators and returned to these same segments after 1.5 years. Surprisingly, we find an “all-or-none” infection clearance response: the most-diseased segments clear if any other lung segment in that person clears, and the least-diseased segments remain infected if others in that person do. Furthermore, neutrophilic inflammation completely resolves where Pa clears but remains elevated where Pa persists. These data indicate that post-modulator infections are not limited to severely diseased segments and that Pa infections drive persistent lung inflammation after modulators.
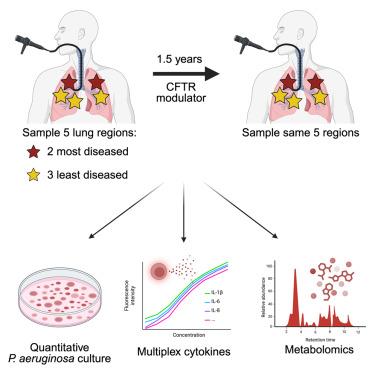
假单胞菌感染持续后CFTR调节剂广泛存在于整个肺部和驱动肺部炎症
囊性纤维化跨膜传导调节剂(CFTR)可改善导致囊性纤维化的生理缺陷,但大多数人的肺部仍然感染和炎症。一个主要的假设暗示受损的肺段是持续感染的原因,并预测个体肺部轻度病变的肺段在治疗后会清除,而严重病变的肺段则不会。我们的发现反驳了这一假设。在使用调剂剂之前,我们使用支气管镜对铜绿假单胞菌(Pa)感染个体中受损最小和最严重的肺段进行采样,并在1.5年后返回这些相同的肺段。令人惊讶的是,我们发现了一种“全有或全无”的感染清除反应:如果这个人的其他肺段清除了,那么患病最严重的肺段也清除了,如果这个人的其他肺段清除了,那么患病最轻的肺段仍然被感染。此外,中性粒细胞炎症在Pa清除的地方完全消退,但在Pa持续存在的地方仍然升高。这些数据表明,调节剂后感染并不局限于严重患病的节段,而且在调节剂后,Pa感染会导致持续的肺部炎症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


