Trehalose-stabilized micelle-in-microparticles of icariin targeting IL-4 pathway in chronic obstructive pulmonary disease
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory airway disorder with limited therapeutic strategies capable of addressing its underlying immunopathology. Icariin (ICA), a flavonoid with documented anti-inflammatory and antioxidant activity, exhibits mechanistic relevance to COPD pathology but is limited in clinical utility due to poor aqueous solubility and low systemic bioavailability. This study reports the development of a dry powder inhalation system comprising DSPE-PEG2000/DPPC micelle-in-microparticles stabilized with trehalose for targeted pulmonary delivery of ICA. Spray-drying produced respirable microparticles with a mass median aerodynamic diameter of 2.36 ± 0.3 µm, an emitted dose of 93 %, and a fine particle fraction of 44 %, consistent with efficient deposition in the lower respiratory tract. Physicochemical characterization confirmed ICA encapsulation in an amorphous state, with preservation of micellar structure upon rehydration, supporting formulation stability. In vitro studies demonstrated enhanced uptake of ICA-loaded micelles by RAW 264.7 macrophages and a 73 % reduction in IL-4-induced CD206 expression, indicative of inhibition of M2 macrophage polarization. These findings support the potential applicability of this inhalable system for modulating macrophage-mediated inflammation in COPD. In vivo studies are required to evaluate pharmacokinetic behaviour, pulmonary distribution, and therapeutic efficacy in validated models of chronic obstructive pulmonary disease.
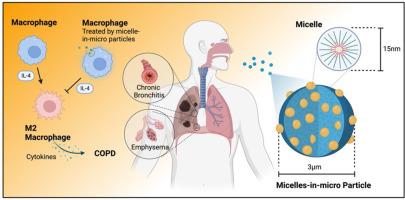
海藻糖稳定的淫羊藿苷微胶束靶向慢性阻塞性肺疾病IL-4通路
慢性阻塞性肺疾病(COPD)是一种进行性炎性气道疾病,其治疗策略有限,无法解决其潜在的免疫病理。淫羊藿苷(ICA)是一种具有抗炎和抗氧化活性的类黄酮,与COPD病理表现出机制相关性,但由于水溶性差和系统生物利用度低,临床应用受到限制。本研究报告了一种干粉吸入系统的开发,该系统由海藻糖稳定的DSPE-PEG2000/DPPC胶束微粒组成,用于靶向肺输送ICA。喷雾干燥产生的可吸入微颗粒的空气动力学直径中位数为2.36 ± 0.3 µm,发射剂量为93%,细颗粒分数为44%,符合下呼吸道的有效沉积。物理化学表征证实了ICA包封在无定形状态下,在再水化时保持了胶束结构,支持配方的稳定性。体外研究表明,RAW 264.7巨噬细胞对ica负载胶束的摄取增强,il -4诱导的CD206表达降低73%,表明M2巨噬细胞极化受到抑制。这些发现支持了这种可吸入系统在调节COPD中巨噬细胞介导的炎症中的潜在适用性。需要进行体内研究来评估慢性阻塞性肺疾病模型的药代动力学行为、肺分布和治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


