Free-energy analysis of cosolvent effects on biomolecular aggregation
IF 2.7
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
A theoretical-computational scheme for analyzing the effect of an added cosolvent on the aggregation equilibrium of a biomolecule is presented. The scheme is based on the relation that the derivative of the excess chemical potential with respect to the cosolvent concentration is determined by the corresponding derivative of the solvation free energy averaged over the solute configurations. The role of solvation is highlighted in the cosolvent-induced shift in the aggregation equilibrium of a biomolecule, and an illustrative analysis with all-atom models is provided for an amyloid peptide by employing the energy-representation method to compute the solvation free energy. Adenosine triphosphate (ATP) and urea are adopted as a cosolvent added to water, and the former is seen to inhibit aggregation more effectively than the latter. The solvation free energy is decomposed into the contributions from intermolecular-interaction components such as electrostatic, van der Waals, and excluded-volume, and it is found that the cosolvent effects are governed by the van der Waals interaction for both of ATP and urea. A theoretical-computational scheme is formulated to analyze the shift in the aggregation equilibrium of a biomolecule upon addition of a cosolvent. The cosolvent-induced change in the solvation free energy plays the central role in the formulation, and it is shown for a model peptide that the ATP and urea cosolvents make the solvent environment more favorable for dissociated monomers than for aggregates. The effect of ATP to inhibit aggregation is brought by van der Waals interactions due to cancellation of the electrostatic effects between ATP and water.
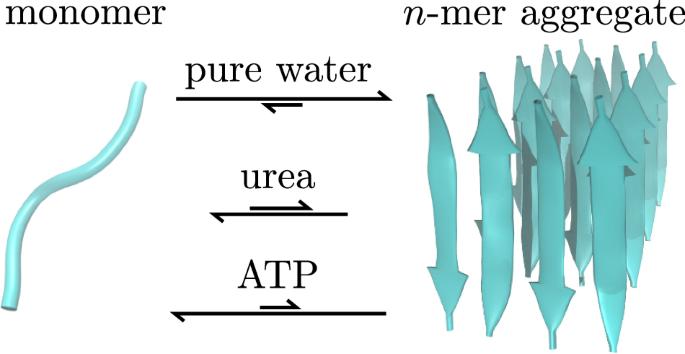
助溶剂对生物分子聚集作用的自由能分析
提出了一种分析添加助溶剂对生物分子聚集平衡影响的理论计算方法。该方案基于这样的关系,即过量化学势对助溶剂浓度的导数由溶质构型上的平均溶剂化自由能的相应导数决定。强调了溶剂化在助溶剂诱导的生物分子聚集平衡转移中的作用,并通过使用能量表示方法计算溶剂化自由能,为淀粉样肽提供了全原子模型的说明性分析。采用三磷酸腺苷(ATP)和尿素作为助溶剂加入水中,前者比后者更有效地抑制聚合。将溶剂化自由能分解为静电、范德华和排除体积等分子间相互作用分量的贡献,发现ATP和尿素的共溶剂效应均受范德华相互作用的支配。本文提出了一种理论计算方法来分析生物分子在加入助溶剂后聚集平衡的变化。助溶剂引起的溶剂化自由能的变化在配方中起着核心作用,对于模型肽,ATP和尿素助溶剂使溶剂环境更有利于解离单体而不是聚集体。ATP抑制聚集的作用是通过范德华相互作用产生的,这是由于ATP和水之间的静电效应被抵消了。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


