Designable synthetic complex coacervates enabling protein delivery to cells
IF 2.7
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
Biomolecular condensates offer a versatile platform for the accumulation of biomacromolecules, particularly proteins. This study investigated synthetic complex coacervates as a model of biomolecular condensates and developed a novel tool for the intracellular delivery of proteins to overcome the issues of cytotoxicity and poor cellular uptake. By optimizing preparation conditions and chemical structures and developing a simple pre-coating method, we achieved an improvement in the interaction and internalization of coacervates for HeLa and Jurkat cells and the reduction of cytotoxicity. Furthermore, the use of charge-density reduced polymers enabled the effective encapsulation and intracellular delivery of various proteins. The mechanism of cellular internalization was also investigated, and macropinocytosis was concluded to be a primary internalization pathway. These findings provide a foundation for future advancements in biomaterials. Complex coacervates (CCs) are a promising platform for protein encapsulation obtained via simple mixing of some components. Using the novel precoating treatment of living cells, the cellular internalization of CCs was greatly enhanced. Besides, upon using the side-chain modified aniomers for CC fabrication, a wide variety of proteins were successfully encapsulated, which can expand the future biomedical application of CCs.
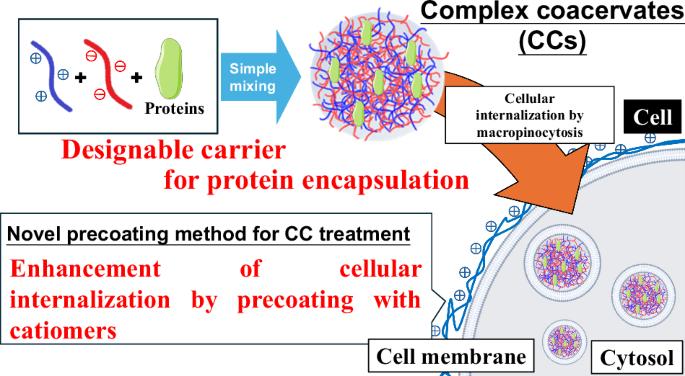
可设计的合成复合物凝聚体,使蛋白质能够传递到细胞
生物分子凝聚体为生物大分子,特别是蛋白质的积累提供了一个通用的平台。本研究研究了合成复杂凝聚体作为生物分子凝聚体的模型,并开发了一种新的细胞内蛋白质递送工具,以克服细胞毒性和细胞摄取不良的问题。通过优化制备条件和化学结构,开发一种简单的预包膜方法,我们改善了HeLa和Jurkat细胞凝聚物的相互作用和内化,降低了细胞毒性。此外,使用电荷密度降低的聚合物使各种蛋白质的有效封装和细胞内递送成为可能。研究了细胞内化的机制,认为巨噬细胞是主要的内化途径。这些发现为未来生物材料的发展奠定了基础。复杂凝聚体(CCs)是一种很有前途的蛋白质包封平台,通过简单的混合得到一些组分。利用新型的活细胞预涂层处理,CCs的细胞内化得到了极大的增强。此外,使用侧链修饰的异构体制备CC,可以成功封装多种蛋白质,这可以扩大CC在未来的生物医学应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


