Tea factory waste as a cost-effective and sustainable Cadmium(II) adsorbent: Investigating adsorption isotherms, kinetics, and thermodynamics
IF 4.1
3区 地球科学
Q2 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
Abstract
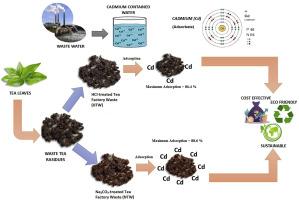
In the quest for sustainable wastewater treatment, the reuse of locally available agro-industrial waste materials presents a promising alternative. Tripura, one of India's major tea-producing states, generates abundant tea factory waste (TFW) that holds significant potential as an affordable adsorbent for heavy metal remediation. This study explores the adsorption efficiency of two chemically treated forms of TFW—Na2CO3-treated (NTW) and HCl-treated (HTW)—for the removal of Cadmium (II) from aqueous solutions. Comprehensive characterization using SEM, FTIR, and EDX revealed enhanced porosity and functional group availability in both materials, particularly NTW. Increasing adsorbent dose (1–3 g/L) improved Cd(II) removal, with NTW and HTW achieving 63.85 % and 61.7 %, respectively. Though percentage removal dropped at higher Cd(II) concentrations, adsorption capacity increased, reaching 85.3 %–94.6 % (NTW) and 82.6 %–92.6 % (HTW). Higher agitation rates further improved removal, peaking at 88.6 % (NTW) and 86.4 % (HTW). Results indicated that NTW achieving a maximum efficiency of 90.08 % under optimal conditions (pH 7, 2 g/L dose, 350 rpm, 318 K, 60 min). The adsorption followed Elovich kinetics and best fit the Langmuir isotherm, confirming monolayer chemisorption. Thermodynamic analysis showed the process to be spontaneous and endothermic, with an activation energy of 76.602 kJ/mol. Using NTW for Cd(II) adsorption, wastewater's chemical oxygen demand (COD) dropped significantly from 138 to 74 mg/L. While the study highlights the low cost (Rs. 7/kg for NTW) and potential circular reuse of TFW, it also recognizes the need for future research to include life cycle assessment (LCA), energy analysis, and regeneration performance.
茶厂废料作为一种具有成本效益和可持续性的镉吸附剂:研究吸附等温线、动力学和热力学
在寻求可持续废水处理的过程中,对当地可用的农业工业废料进行再利用是一个很有前途的选择。特里普拉邦是印度主要的产茶邦之一,产生了大量的茶厂废物(TFW),这些废物作为重金属修复的吸附剂具有巨大的潜力。本研究探讨了两种化学处理形式的tfw - na2co3处理(NTW)和盐酸处理(HTW) -对水溶液中镉(II)的吸附效率。通过SEM、FTIR和EDX的综合表征,两种材料的孔隙度和官能团可用性都有所提高,尤其是NTW。增加吸附剂剂量(1 ~ 3 g/L)可提高对Cd(II)的去除率,其中NTW和HTW的去除率分别达到63.85%和61.7%。Cd(II)浓度越高,去除率越低,吸附量越大,分别达到85.3% ~ 94.6% (NTW)和82.6% ~ 92.6% (HTW)。更高的搅拌速率进一步提高了去除率,最高达到88.6% (NTW)和86.4% (HTW)。结果表明,在最佳条件(pH 7, 2 g/L剂量,350 rpm, 318 K, 60 min)下,NTW的最高效率为90.08%。吸附符合Elovich动力学,最符合Langmuir等温线,证实了单层化学吸附。热力学分析表明,该反应为自发的吸热反应,活化能为76.602 kJ/mol。利用NTW吸附Cd(II),废水的化学需氧量(COD)由138 mg/L显著下降至74 mg/L。虽然该研究强调了NTW的低成本(每公斤7卢比)和潜在的循环再利用,但它也认识到未来的研究需要包括生命周期评估(LCA)、能源分析和再生性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physics and Chemistry of the Earth
地学-地球科学综合
CiteScore
5.40
自引率
2.70%
发文量
176
审稿时长
31.6 weeks
期刊介绍:
Physics and Chemistry of the Earth is an international interdisciplinary journal for the rapid publication of collections of refereed communications in separate thematic issues, either stemming from scientific meetings, or, especially compiled for the occasion. There is no restriction on the length of articles published in the journal. Physics and Chemistry of the Earth incorporates the separate Parts A, B and C which existed until the end of 2001.
Please note: the Editors are unable to consider submissions that are not invited or linked to a thematic issue. Please do not submit unsolicited papers.
The journal covers the following subject areas:
-Solid Earth and Geodesy:
(geology, geochemistry, tectonophysics, seismology, volcanology, palaeomagnetism and rock magnetism, electromagnetism and potential fields, marine and environmental geosciences as well as geodesy).
-Hydrology, Oceans and Atmosphere:
(hydrology and water resources research, engineering and management, oceanography and oceanic chemistry, shelf, sea, lake and river sciences, meteorology and atmospheric sciences incl. chemistry as well as climatology and glaciology).
-Solar-Terrestrial and Planetary Science:
(solar, heliospheric and solar-planetary sciences, geology, geophysics and atmospheric sciences of planets, satellites and small bodies as well as cosmochemistry and exobiology).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


