The catalytic effect of silver on acidic ferric-sulfate leaching of chalcopyrite: A microscopic cyclic reaction
IF 8.9
1区 地球科学
Q1 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
Abstract
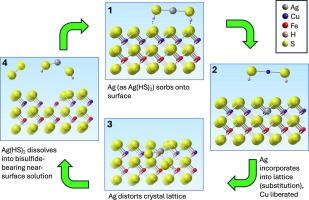
Copper extraction from chalcopyrite is challenging, because acid dissolution is slow, occurring incongruently via a complex three-step reaction mechanism. Silver has been known to catalyse copper extraction from chalcopyrite since the 1970's; yet the mechanism remains controversial. Microcharacterisation of experimental products obtained under optimal leaching conditions (50–150 μm chalcopyrite grains in ferric/ferrous-sulfate solution with a redox potential around 500 mV vs. Ag/AgCl, approximately 1ppm Ag; [Ag] 6.4 × 10−6 mol/L; 70 °C; 4 days) highlights the heterogeneity of the reaction: µm-thick layers of a porous copper-sulfide with variable composition formed both in cracks within, and on the surface of the chalcopyrite grains. There is no evidence for formation of Ag-rich phases (Ag2S(s), Ag0(s)). The fundamental three-step reaction mechanism remains the same with or without added silver; silver merely accelerates the initial dissolution step.
An integrated model for the catalytic effect of silver is proposed that incorporates recent advances in the reactivity of sulfide minerals. The initial reaction follows a ‘Fluid-Induced Solid State Diffusion Mechanism’, where diffusion of Fe in the chalcopyrite lattice is driven towards the surface by its rapid removal into solution, resulting in a Fe-deficient surface layer. The large Ag+ ion, relative to Cu+/Fe3+, diffuses into this Fe-deficient surface layer and accelerates chalcopyrite dissolution in the subsequent step, whereby chalcopyrite is replaced by copper sulfides via an interface coupled dissolution reprecipitation reaction as a consequence of the sulfide-rich micro-environment at the mineral surface. Effective Ag+ recycling is key to the catalytic effect of silver, and occurs as a result of the strong affinity of Ag+ for bisulfide ligands accumulating at the surface of dissolving chalcopyrite.
银对酸性硫酸铁浸出黄铜矿的催化作用:微观循环反应
从黄铜矿中提取铜具有挑战性,因为酸溶缓慢,通过一个复杂的三步反应机制不一致地发生。自20世纪70年代以来,人们就知道银可以催化从黄铜矿中提取铜;然而,这一机制仍存在争议。在最佳浸出条件下获得的实验产品的微观表征(50-150 μm黄铜矿颗粒在铁/硫酸亚铁溶液中,氧化还原电位约为500 mV vs. Ag/AgCl,约为1ppm Ag;[Ag] 6.4 × 10−6 mol/L;70°C;4天)强调了反应的非均质性:在黄铜矿颗粒内部和表面的裂缝中形成了μ m厚的多孔硫化铜层,其组成各不相同。没有证据表明形成富银相(Ag2S(s), Ag0(s))。无论是否添加银,基本的三步反应机理保持不变;银只是加速了最初的溶解步骤。结合硫化物矿物反应性的最新进展,提出了银催化作用的综合模型。最初的反应遵循“流体诱导的固态扩散机制”,其中黄铜矿晶格中的铁通过快速移除到溶液中而向表面扩散,导致表面层缺铁。相对于Cu+/Fe3+,较大的Ag+离子扩散到缺铁的表层,加速了后续步骤中黄铜矿的溶解,由于矿物表面富含硫化物的微环境,黄铜矿通过界面耦合溶解再沉淀反应被硫化物铜取代。Ag+的有效回收是银的催化作用的关键,这是由于Ag+对溶解的黄铜矿表面积累的二硫化物配体具有很强的亲和力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Geoscience frontiers
Earth and Planetary Sciences-General Earth and Planetary Sciences
CiteScore
17.80
自引率
3.40%
发文量
147
审稿时长
35 days
期刊介绍:
Geoscience Frontiers (GSF) is the Journal of China University of Geosciences (Beijing) and Peking University. It publishes peer-reviewed research articles and reviews in interdisciplinary fields of Earth and Planetary Sciences. GSF covers various research areas including petrology and geochemistry, lithospheric architecture and mantle dynamics, global tectonics, economic geology and fuel exploration, geophysics, stratigraphy and paleontology, environmental and engineering geology, astrogeology, and the nexus of resources-energy-emissions-climate under Sustainable Development Goals. The journal aims to bridge innovative, provocative, and challenging concepts and models in these fields, providing insights on correlations and evolution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


