Electrochemiluminescent detection of glucose by employing a cobalt-based stainless steel electrode
IF 4.5
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Electrodes employed in the detection of glucose are commonly fabricated from conductive substances, including platinum, gold, or carbon, often modified with enzymes such as glucose oxidase, and are designed to promote electrochemical interactions that precisely quantify glucose levels in biological specimens. Cobalt-based stainless steel exhibits excellent biocompatibility and corrosion resistance and has been using in various advanced applications, such as medical devices. Herein, we demonstrate the first electroanalytical application of cobalt-based stainless steel electrode through its application for luminol electrochemiluminescence (ECL) detection of hydrogen peroxide and glucose. Luminol‑hydrogen peroxide shows intense ECL at cobalt-based stainless steel electrode, enabling the detection of H₂O₂ in the concentration range of 0.01 μM to 20 μM with a limit of detection of 1.25 nM. Moreover, intense ECL of luminol‑hydrogen peroxide at cobalt-based stainless steel electrode was used to develop an ECL glucose detection method by coupling with the enzymatic conversion of glucose to hydrogen peroxide in the presence of glucose oxidase. The developed ECL glucose detection method shows a wide linear range of 0.005 to 5 mM with a limit of detection of 0.005 mM and a limit of quantification as 0.016 mM, with excellent reproducibility (3.7 % RSD). This method was further validated through its application to real honey samples, and the results were compared by the HPLC method, showcasing its potential for glucose detection. This study implies significant potential of cobalt-based stainless steel electrode across various electroanalytical applications due to its sensitivity, wide detection range, and robustness.
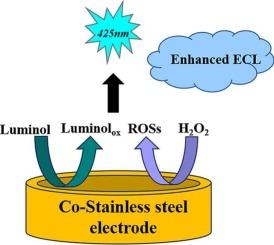
采用钴基不锈钢电极的葡萄糖电化学发光检测
用于葡萄糖检测的电极通常由导电物质制成,包括铂、金或碳,通常用葡萄糖氧化酶等酶修饰,旨在促进电化学相互作用,精确量化生物标本中的葡萄糖水平。钴基不锈钢具有优异的生物相容性和耐腐蚀性,已被用于各种先进的应用,如医疗设备。在此,我们通过将钴基不锈钢电极应用于发光氨电化学发光(ECL)检测过氧化氢和葡萄糖,展示了钴基不锈钢电极的第一个电分析应用。鲁米诺-过氧化氢在钴基不锈钢电极上表现出强烈的ECL,可以在0.01 μM ~ 20 μM的浓度范围内检测到H₂O₂,检测限为1.25 nM。此外,在钴基不锈钢电极上,利用鲁米诺-过氧化氢的强ECL,结合葡萄糖氧化酶存在下葡萄糖转化为过氧化氢,建立了ECL葡萄糖检测方法。所建立的ECL葡萄糖检测方法线性范围为0.005 ~ 5 mM,检测限为0.005 mM,定量限为0.016 mM,重现性好(RSD为3.7%)。将该方法应用于实际蜂蜜样品中进行验证,并与HPLC法进行比较,显示了该方法在葡萄糖检测中的潜力。该研究表明,钴基不锈钢电极具有灵敏度高、检测范围广、坚固耐用等优点,在各种电分析应用中具有重要的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


