Non-invasive detection of protein aggregation in biopharmaceuticals using TD-NMR and MRI
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-08-05
DOI:10.1016/j.ejpb.2025.114830
引用次数: 0
Abstract
With the rapid expansion of biotechnological drugs, ensuring their quality has become essential. Proper storage and transportation are critical to maintaining drug stability and efficacy. This study investigates protein aggregation, a major quality concern in biopharmaceuticals, using low-field NMR, Time Domain NMR and Magnetic Resonance Imaging (MRI), as a non-invasive, rapid alternative to conventional analytical methods. For the first time, MRI, T2 relaxation times, and T2 relaxation spectra were applied to detect aggregation in Humulin R, a low-concentration subcutaneous insulin. Aggregation was induced by heat and agitation to simulate improper handling. MRI effectively distinguished control and stressed samples, while T2 relaxation measurements differentiated stress conditions. Additionally, T2-Inverse Laplace Transform (ILT) indicated aggregate size, aligning with particle size analysis and validating the novel approach. These findings highlight the potential of low-field NMR for biopharmaceutical quality assessment.
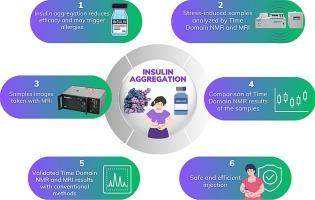
利用TD-NMR和MRI无创检测生物制药中蛋白质聚集
随着生物技术药物的迅速发展,确保其质量变得至关重要。适当的储存和运输对保持药物的稳定性和有效性至关重要。本研究利用低场核磁共振、时域核磁共振和磁共振成像(MRI)作为传统分析方法的非侵入性、快速替代方法,研究了生物制药中一个主要的质量问题——蛋白质聚集。首次应用MRI、T2弛豫时间和T2弛豫谱检测低浓度皮下胰岛素Humulin R的聚集。通过加热和搅拌引起聚集,模拟不适当的处理。MRI可有效区分对照和应力样品,而T2松弛测量可区分应力条件。此外,t2 -拉普拉斯逆变换(ILT)显示了骨料粒度,与粒度分析一致,验证了新方法。这些发现突出了低场核磁共振在生物制药质量评估中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


