Navigating liver toxicity in the age of novel oncological agents
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
The advent of novel oncological therapies, including immune checkpoint inhibitors, antibody-drug conjugates, and protein kinase inhibitors, has revolutionised cancer treatment by significantly improving patient survival across a range of malignancies. However, these advances have been accompanied by the emergence of new and often unpredictable adverse events, among which hepatotoxicity represents a growing clinical challenge. In this review, we provide a comprehensive synthesis of current knowledge on liver injury associated with these three key classes of oncological agents, with a particular focus on mechanisms of action and hepatotoxicity, clinical presentation, and management strategies. Given the expanding use of these agents, both as monotherapies or in combination regimens, this topic is of pressing relevance to hepatologists and oncologists alike. As combination therapies become increasingly common, the complexity of drug–liver interactions and their implications for patient safety demand greater interdisciplinary awareness and collaboration. This review advocates for a pragmatic approach to the management of drug-induced liver injury in patients with cancer, underscoring the critical need to balance hepatic preservation with the imperative of maintaining oncological efficacy in this uniquely vulnerable population. By addressing an emerging area of clinical importance, we aim to stimulate further research on oncological hepatotoxicity, a phenomenon that is poised to become increasingly prevalent in routine clinical practice.
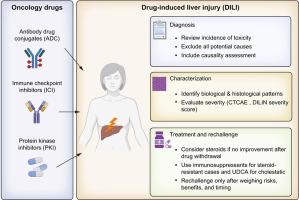
在新的肿瘤药物时代导航肝毒性
新型肿瘤疗法的出现,包括免疫检查点抑制剂、抗体-药物偶联物和蛋白激酶抑制剂,通过显著提高一系列恶性肿瘤患者的生存率,已经彻底改变了癌症治疗。然而,这些进步伴随着新的和不可预测的不良事件的出现,其中肝毒性是一个越来越大的临床挑战。在这篇综述中,我们提供了与这三种主要肿瘤药物相关的肝损伤的最新知识的综合,特别关注作用机制和肝毒性,临床表现和管理策略。鉴于这些药物的使用日益扩大,无论是作为单一疗法还是联合疗法,这个话题对肝病学家和肿瘤学家都具有紧迫的相关性。随着联合治疗变得越来越普遍,药物-肝脏相互作用的复杂性及其对患者安全的影响需要更多的跨学科意识和合作。这篇综述提倡一种务实的方法来管理癌症患者的药物性肝损伤,强调在这一独特的易感人群中,肝脏保护与维持肿瘤疗效之间的平衡至关重要。通过解决一个具有临床重要性的新兴领域,我们的目标是刺激对肿瘤肝毒性的进一步研究,这一现象在常规临床实践中越来越普遍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


