Affinity of rhizobacterial chitinases towards chitin polymers from shrimp exoskeleton and fungal cell wall: Optimization for effective biocontrol potentials
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-07-25
DOI:10.1016/j.carpta.2025.100959
引用次数: 0
Abstract
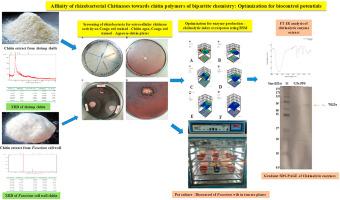
Bacteria that can hydrolyze shrimp shell chitin and fungal cell wall chitin were isolated from tomato rhizosphere soil in an effort to find chitin-degrading enzymes. Extracellular nature of chitinase was confirmed in agarose-chitin. Chitin from virulent Fusarium oxysporum f. sp. lycopersici was characterized using XRD to study crystallinity, and the presence chitin - β-glucan conjugates. Chitinase production was optimized through RSM using shrimp chitin and fungal chitin as substrates independently for temperature, pH, incubation time, and substrate concentration and statistically validated. Crude exoproteins from Achromobacter mucicolens showed increased chitinolytic index and antifungal effects against three target fungi Fusarium oxysporum f.sp. lycopersici, Fusarium equiseti and Sarocladium sp. In vivo pot culture of treated tomato seedlings observes appreciable biocontrol effects of chitinolytic rhizobacterial consortium during the late stages of vascular wilt infections. Results conclude that the rhizosphere bacteria produced chitinases preferably show effective chitinolytic index for fungal cell wall chitin than shrimp shell chitin at optimum conditions for enzyme catalysis observed in our study. Secreted chitinases possess molecular mass approximately 70 KDa classifying the enzymes into bacterial chitinases belongs to family 18 of glycosyl hydrolases. Our study observing the chitin substrate preference by rhizobacterial chitinases stresses their role in biocontrol.
根瘤菌几丁质酶对虾外骨骼和真菌细胞壁几丁质聚合物的亲和力:有效生物防治潜力的优化
从番茄根际土壤中分离出能水解虾壳几丁质和真菌细胞壁几丁质的细菌,寻找几丁质降解酶。几丁质酶的胞外性质在琼脂糖-几丁质中得到证实。利用x射线衍射(XRD)研究了番茄枯萎菌(Fusarium oxysporum f. sp. lycopersici)中甲壳素的结晶度,以及甲壳素- β-葡聚糖缀合物的存在。以对虾几丁质和真菌几丁质为底物,分别以温度、pH、孵育时间和底物浓度为条件,通过RSM优化几丁质酶的生产,并进行了统计学验证。mucicolens无色杆菌的粗外蛋白对3种目标真菌尖孢镰刀菌的几丁质溶解指数和抑菌作用均有显著提高。在番茄幼苗体内盆栽培养中,几丁质溶解根瘤菌联合体在血管性枯萎病后期有明显的生物防治效果。结果表明,在本研究观察到的最佳酶催化条件下,根际细菌产生的几丁质酶对真菌细胞壁几丁质的水解指数优于对虾壳几丁质。分泌的几丁质酶分子量约为70 KDa,属细菌几丁质酶,属糖基水解酶家族18。本研究观察了根细菌几丁质酶对几丁质底物的偏好,强调了它们在生物防治中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


