Calmodulin enhancement of mitochondrial calcium uniporter function in isolated mitochondria
IF 4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Mitochondrial calcium (Ca2+) uptake and factors that regulate this process have been an area of immense interest given the roles in cellular energetics. Here, we have investigated the ability of the Ca2+ sensing protein Calmodulin (CaM) to modify the function of the Mitochondrial Ca2+ Uniporter (MCU). Our data leveraged recombinantly produced CaM and mitochondria isolated from healthy and MCU impaired/diseased mice (Barth syndrome model). We found CaM enhanced Ca2+ uptake in both the absence and presence of CaMKII inhibition (KN93 as well as AIP). Mitochondria lacking function MCU (Barth syndrome model) validated that MCU was responsible for Ca2+ uptake in our experiments. Control experiments demonstrate that the observed CaM enhancement does not arise from CaM Ca2+ buffering. Fitting the Ca2+fluorescence data supported a monophasic decay process where the presence of CaM yielded enhanced kinetic rates of Ca2+ uptake. This CaM enhancement effect persisted in the presence of PTP impairment (cyclosporin), and subtle modification to the CaM protein sequence (D131E) revealed that an intact CaM-C domain Ca2+ binding was required for enhancement of MCU function.
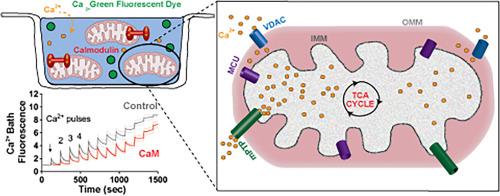
钙调素增强离体线粒体钙转运蛋白功能
线粒体钙(Ca2+)摄取和调节这一过程的因素一直是一个非常感兴趣的领域,因为它在细胞能量学中的作用。在这里,我们研究了Ca2+传感蛋白钙调蛋白(CaM)改变线粒体Ca2+单转运蛋白(MCU)功能的能力。我们的数据利用了从健康和MCU受损/患病小鼠(Barth综合征模型)中分离的重组产生的CaM和线粒体。我们发现CaM在CaMKII抑制(KN93和AIP)缺失和存在的情况下都能增强Ca2+摄取。线粒体缺乏功能MCU (Barth综合征模型)在我们的实验中证实了MCU负责Ca2+摄取。对照实验表明,观察到的CaM增强不是由CaM Ca2+缓冲引起的。拟合Ca2+荧光数据支持单相衰减过程,其中CaM的存在产生增强的Ca2+摄取的动力学速率。这种CaM增强效应在PTP损伤(环孢素)存在时持续存在,并且对CaM蛋白序列(D131E)的细微修饰表明,增强MCU功能需要完整的CaM- c结构域Ca2+结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Cell calcium
生物-细胞生物学
CiteScore
8.70
自引率
5.00%
发文量
115
审稿时长
35 days
期刊介绍:
Cell Calcium covers the field of calcium metabolism and signalling in living systems, from aspects including inorganic chemistry, physiology, molecular biology and pathology. Topic themes include:
Roles of calcium in regulating cellular events such as apoptosis, necrosis and organelle remodelling
Influence of calcium regulation in affecting health and disease outcomes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


