Synthesis and mechanistic study of a CuO/Cu2(OH)2CO3 catalyst for rapid peroxymonosulfate activation to efficiently degrade 17α-ethynylestradiol
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The combination of peroxymonosulfate based advanced oxidation processes (PMS-AOPs) with membrane separation technology has shown considerable potential for the degradation of organic pollutants in wastewater. Herein, a CuO/Cu2(OH)2CO3 composite was synthesized in one step using a microwave-assisted method and employed to activate PMS for the degradation of 17α-ethynylestradiol (EE2)—a common environmental endocrine disruptor—in water. The associated CuO/Cu2(OH)2CO3–PMS system removed 98.13 % of EE2 (3 mg/L) within 8 min, substantially outperforming CuO and Cu2(OH)2CO3 in terms of degradation efficiency. The EE2 removal efficiency of the CuO/Cu2(OH)2CO3–PMS system remained at 95.52 % after five cycles, demonstrating excellent stability. Moreover, this system exhibited good degradation ability for various types of pollutants (Congo red, methylene blue, rhodamine B, and tetracycline hydrochloride). Mechanistic studies indicated that Cu ions on the surface of the CuO/Cu2(OH)2CO3 composite and those partially leached into the associated solution heterogeneously and homogeneously catalyzed PMS activation, respectively; this resulted in the formation of a Cu2+/Cu+ redox cycle and generation of a series of highly oxidative radicals (•OH, SO4•−, and •O2−) and nonradicals (1O2), which cooperatively and efficiently degraded EE2 through multiple pathways. Three possible EE2 degradation pathways were analyzed, and the toxicities of the intermediate products were evaluated. Overall, this research provides fundamental insights into the catalytic mechanism of CuO/Cu2(OH)2CO3 for PMS activation and lays the groundwork for future integration into continuous-flow systems for practical wastewater treatment applications.
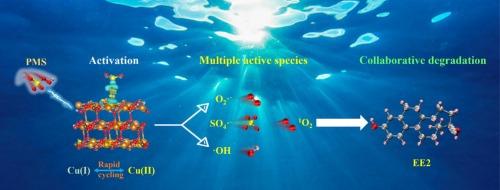
快速过氧单硫酸盐活化CuO/Cu2(OH)2CO3催化剂的合成及高效降解17α-乙基雌二醇的机理研究
以过氧单硫酸盐为基础的高级氧化工艺(PMS-AOPs)与膜分离技术相结合,在废水中有机污染物的降解方面显示出相当大的潜力。本文采用微波辅助法一步合成CuO/Cu2(OH)2CO3复合物,并激活PMS降解水中常见的环境内分泌干扰物17α-乙炔雌醇(EE2)。结合的CuO/Cu2(OH)2CO3 - pms体系在8分钟内去除了98.13%的EE2 (3 mg/L),在降解效率方面大大优于CuO和Cu2(OH)2CO3。经过5次循环后,CuO/Cu2(OH) 2CO3-PMS体系的EE2去除率仍保持在95.52%,具有良好的稳定性。此外,该体系对各种类型的污染物(刚果红、亚甲蓝、罗丹明B和盐酸四环素)具有良好的降解能力。机理研究表明,CuO/Cu2(OH)2CO3复合材料表面的Cu离子和部分浸出到伴生溶液中的Cu离子分别具有非均相和均相催化PMS活化的作用;这导致形成Cu2+/Cu+氧化还原循环,并产生一系列高氧化自由基(•OH, SO4•−和•O2−)和非自由基(1O2),这些自由基通过多种途径协同有效地降解EE2。分析了三种可能的EE2降解途径,并对中间产物的毒性进行了评价。总的来说,本研究为CuO/Cu2(OH)2CO3催化PMS活化的机制提供了基本的见解,并为未来集成到实际废水处理应用的连续流系统奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


