Associations of indoor airborne microbiome with systemic inflammation in the context of indoor particulate matter pollution and the metabolic mechanisms
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Microorganisms constitute an essential component in the indoor environment, which is closely related to human health. However, there is limited evidence regarding the associations between indoor airborne microbiome and systemic inflammation, as well as whether this association is modified by indoor particulate matter and the underlying mechanisms. In this prospective repeated-measure study among 66 participants, indoor airborne microbiome was characterized using amplicon sequencing and qPCR. Indoor fine particulate matter (PM2.5) and inhalable particulate matter (PM10) were measured. Systemic inflammatory biomarkers were assessed, including white blood cell (WBC), neutrophil (NEUT), monocyte, eosinophil counts, and their proportions. Targeted serum amino acid metabolomics were conducted to explore the underlying mechanisms. Linear mixed-effect models revealed that bacterial and fungal Simpson diversity were significantly associated with decreased WBC and NEUT. For example, for each interquartile range increase in the bacterial Simpson diversity, WBC and NEUT changed by -4.53 % (95 % CI: -8.25 %, -0.66 %) and -5.95 % (95 % CI: -11.3 %, -0.27 %), respectively. Notably, increased inflammatory risks of airborne microbial exposure were observed when indoor PM2.5 and PM10 levels were below the WHO air quality guidelines. Mediation analyses indicated that dopamine metabolism partially mediated the anti-inflammatory effects of fungal diversity exposure. Overall, our study indicated protection from a diverse indoor microbial environment on cardiovascular health and proposed an underlying mechanism through amino acid metabolism. Additionally, health risks associated with microbial exposure deserve more attention in contexts of low indoor particulate matter pollution. Further research is necessary to fully disentangle the complex relationships between indoor microbiome, air pollutants, and human health.
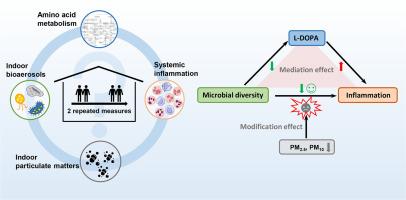
室内颗粒物污染环境下室内空气微生物群与全身性炎症的关系及其代谢机制
微生物是室内环境的重要组成部分,与人体健康密切相关。然而,关于室内空气微生物群与全身性炎症之间的关联,以及这种关联是否被室内颗粒物改变及其潜在机制的证据有限。在这项前瞻性重复测量研究中,66名参与者使用扩增子测序和qPCR对室内空气微生物组进行了表征。测量室内细颗粒物(PM2.5)和可吸入颗粒物(PM10)。评估全身炎症生物标志物,包括白细胞(WBC)、中性粒细胞(NEUT)、单核细胞、嗜酸性粒细胞计数及其比例。通过靶向血清氨基酸代谢组学研究其潜在机制。线性混合效应模型显示,细菌和真菌Simpson多样性与WBC和NEUT的降低显著相关。例如,细菌Simpson多样性每增加四分位数,WBC和NEUT分别变化- 4.53% (95% CI: - 8.25%, - 0.66%)和- 5.95% (95% CI: - 11.3%, - 0.27%)。值得注意的是,当室内PM2.5和PM10水平低于世卫组织空气质量指南时,观察到空气中微生物暴露的炎症风险增加。中介分析表明,多巴胺代谢在一定程度上介导了真菌多样性暴露的抗炎作用。总的来说,我们的研究表明,不同的室内微生物环境对心血管健康的保护作用,并提出了通过氨基酸代谢的潜在机制。此外,在室内颗粒物污染较低的情况下,与微生物接触有关的健康风险值得更多关注。进一步研究室内微生物群、空气污染物和人体健康之间的复杂关系是必要的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


