Smart zwitterionic biodegradable hydrogel for sustained peptide delivery: Application to the neurotherapeutic peptide NX210c
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-04
DOI:10.1016/j.bioadv.2025.214437
引用次数: 0
Abstract
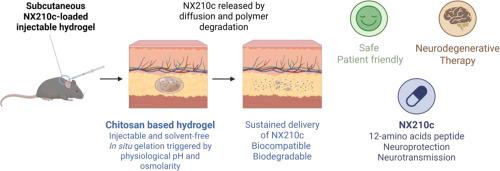
Therapeutic peptides offer a promising balance between specificity, safety, and bioavailability. However, their clinical use is limited by poor in vivo stability and short half-life, requiring frequent administrations that can compromise patient compliance and therapeutic outcomes. NX210c, a synthetic cyclic dodecapeptide derived from the thrombospondin repeat motif of the sub-commissural organ-spondin shows neuroprotective effects, making it a promising candidate for the treatment of neurological disorders. However, its short half-life (~15 min) requires intravenous infusion highlighting the interest of an alternative delivery method to improve the quality of life for patients.
This study, report the preclinical development of an injectable, biodegradable chitosan-based hydrogel functionalized with the macrocyclic ligand DOTAGA, designed for the sustained subcutaneous release of NX210c. The hydrogel forms in situ under physiological conditions, via electrostatic and hydrogen bonding interactions. The solvent-free formulation is simple and clinically compatible. Hydrogel candidate were screened in vitro for injectability, gelation, peptide loading, and release kinetics. In vivo validation on rodents confirmed subcutaneous injectability, gelation ability, biocompatibility and biodegradability within few weeks. Pharmacokinetics studies demonstrated distinct profiles: intravenous injection led to peptide clearance within 1 h; free NX210c administered subcutaneously slightly prolonged its presence in the bloodstream, with complete elimination observed within approximately 3 h. The use of hydrogel, enables AUCs dozens of times higher, with peptide levels sustained for over 10 to 15 days. These results highlight the hydrogel's potential as a patient-friendly, clinically translatable platform for therapeutic peptides requiring sustained release. Future studies will focus on the evaluation of its therapeutic efficiency.
用于持续肽递送的智能两性离子可生物降解水凝胶:用于神经治疗肽NX210c
治疗性多肽在特异性、安全性和生物利用度之间提供了一个有希望的平衡。然而,它们的临床使用受到体内稳定性差和半衰期短的限制,需要频繁给药,可能会损害患者的依从性和治疗结果。NX210c是一种合成的环十二肽,它来自于关节下器官反应蛋白的血栓反应蛋白重复基序,具有神经保护作用,使其成为治疗神经系统疾病的有希望的候选者。然而,它的半衰期短(~15分钟)需要静脉输注,这突出了一种替代给药方法的兴趣,以改善患者的生活质量。本研究报告了一种可注射的、可生物降解的、以大环配体DOTAGA为功能化的壳聚糖为基础的水凝胶的临床前开发,该水凝胶旨在持续皮下释放NX210c。水凝胶在生理条件下通过静电和氢键相互作用在原位形成。无溶剂配方简单,临床相容性好。体外筛选候选水凝胶的可注射性、凝胶性、肽负载和释放动力学。在啮齿类动物的体内验证中,几周内证实了皮下注射性、凝胶性、生物相容性和生物降解性。药代动力学研究显示了不同的特征:静脉注射导致肽在1小时内清除;皮下注射游离NX210c可略微延长其在血液中的存在时间,约3小时内观察到完全消除。使用水凝胶可使auc提高数十倍,肽水平持续超过10至15天。这些结果突出了水凝胶作为一种患者友好的、临床可翻译的治疗肽需要持续释放的平台的潜力。今后的研究将着重于对其治疗效果的评价。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


