A versatile antibody capture system drives specific in vivo delivery of mRNA-loaded lipid nanoparticles
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
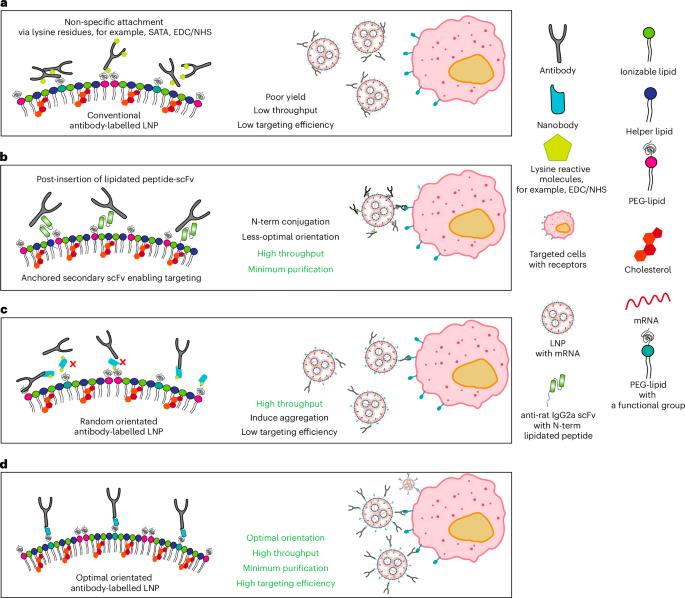
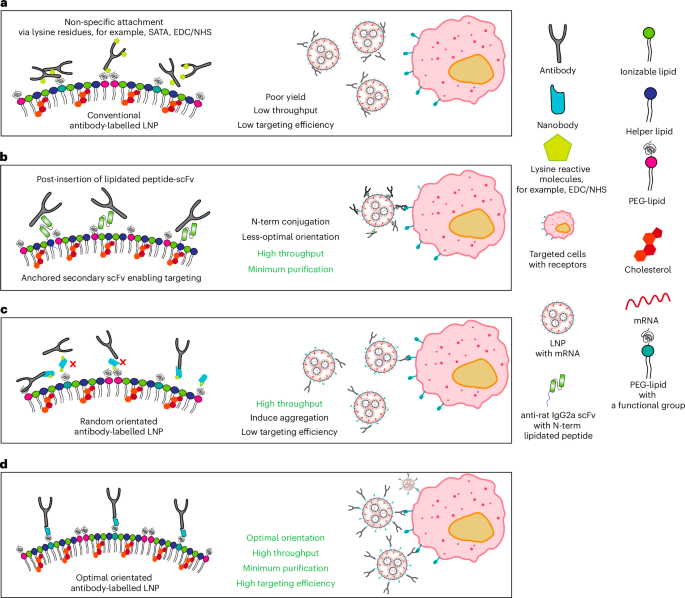
Efficient and precise delivery of mRNA is critical to advance mRNA therapies beyond their current use as vaccines. Lipid nanoparticles (LNPs) efficiently encapsulate and protect mRNA, but non-specific cellular uptake may lead to off-target delivery and minimal delivery to target cells. Functionalizing LNPs with antibodies enables targeted mRNA delivery, but traditional modification techniques require complex conjugation and purification, which often reduces antibody affinity. Here we present a simple method for capturing antibodies in their optimal orientation on LNPs, without antibody modification or complex purification. This strategy uses an optimally oriented anti-Fc nanobody on the LNP surface to capture antibodies, resulting in protein expression levels more than 1,000 times higher than non-targeted LNPs and more than 8 times higher than conventional antibody functionalization techniques. These precisely targeted LNPs showed highly efficient in vivo targeting to T cells, with minimal delivery to other immune cells. This approach enables the rapid development of targeted LNPs and has the potential to broaden the use of mRNA therapies. A mix-and-go system enables the rapid prototyping of antibody formulations, allowing control of their orientation on the surface of LNPs for specific cell targeting, significantly improving the ex vivo and in vivo deliveries of mRNA.
多功能抗体捕获系统驱动特定的体内递送mrna负载脂质纳米颗粒
有效和精确地递送mRNA对于推进mRNA治疗至关重要,使其超越目前作为疫苗的用途。脂质纳米颗粒(LNPs)可以有效地包裹和保护mRNA,但非特异性细胞摄取可能导致脱靶递送和对靶细胞的最小递送。用抗体功能化LNPs可以实现mRNA的靶向递送,但传统的修饰技术需要复杂的偶联和纯化,这往往会降低抗体的亲和力。在这里,我们提出了一种简单的方法来捕获抗体在LNPs上的最佳取向,而不需要抗体修饰或复合物纯化。该策略利用LNP表面的最佳定向抗fc纳米体捕获抗体,导致蛋白质表达水平比非靶向LNP高1000倍以上,比传统抗体功能化技术高8倍以上。这些精确靶向的LNPs在体内显示出对T细胞的高效靶向,而对其他免疫细胞的递送最少。这种方法能够快速开发靶向LNPs,并有可能扩大mRNA治疗的使用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


