Amino acid competition shapes Acinetobacter baumannii gut carriage
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Asymptomatic colonization is often critical for persistence of antimicrobial-resistant pathogens, such as Acinetobacter baumannii, and can increase the risk of clinical infections. However, the ecological factors shaping A. baumannii gut colonization remain unclear. We show that A. baumannii and other pathogenic Acinetobacter evolved to utilize the amino acid ornithine, a non-preferred carbon source, to compete with resident microbiota and persist in the gut in mice. A. baumannii encodes ornithine succinyltransferase (AstO) necessary for catabolizing ornithine, especially in conditions of increased microbial diversity. Supplemental dietary ornithine promotes long-term fecal shedding of A. baumannii. By contrast, supplementation of preferred carbon sources—monosodium glutamate or histidine—abolishes the requirement for ornithine catabolism. Additionally, A. baumannii gut carriage is higher in formula-fed human infants, who generally consume higher levels of protein, revealing dietary impacts on Acinetobacter colonization. Together, these results reveal that ornithine catabolism facilitates A. baumannii colonization, providing a reservoir for pathogen spread.
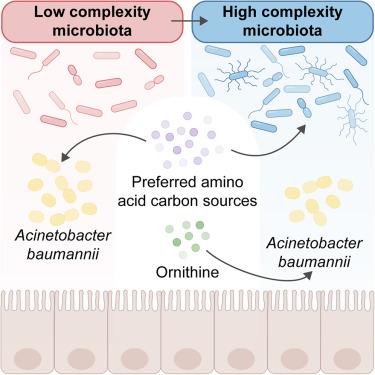
氨基酸竞争塑造鲍曼不动杆菌肠道运输
无症状定植通常对耐药病原体(如鲍曼不动杆菌)的持续存在至关重要,并可增加临床感染的风险。然而,形成鲍曼不动杆菌肠道定植的生态因素仍不清楚。我们发现鲍曼不动杆菌和其他致病性不动杆菌进化到利用氨基酸鸟氨酸(一种非首选碳源)与常驻微生物群竞争并在小鼠肠道中持续存在。鲍曼不动杆菌编码鸟氨酸琥珀基转移酶(AstO),这是鸟氨酸分解代谢所必需的,特别是在微生物多样性增加的条件下。补充鸟氨酸可促进鲍曼不动杆菌的长期粪便排出。相比之下,补充首选的碳源——谷氨酸钠或组氨酸——可以消除鸟氨酸分解代谢的需要。此外,鲍曼不动杆菌肠道携带量在配方奶喂养的人类婴儿中更高,他们通常消耗更高水平的蛋白质,这揭示了饮食对不动杆菌定植的影响。总之,这些结果表明鸟氨酸分解代谢促进了鲍曼不动杆菌的定植,为病原体的传播提供了一个储存库。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


