Biofilm-triggered interfacial assembly of dual-porphyrin heterojunctions for chemo-/sonodynamic treatment of pyomyositis
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Sonodynamic therapy (SDT) indicates advantages in combating antibiotics-resistant bacteria and deep tissue infections, but challenges remain in the development of highly efficient, infection-responsive, and biocompatible sonosensitizers. Herein, tetrakis (4-hydroxyphenyl) porphyrin (TH) and tetrakis (4-carboxyphenyl) zinc porphyrin (ZnTC) are proposed to construct dual-porphyrin heterojunctions (TH/ZnTC), and their matched interface and strong interfacial electric field (IEF) enhance charge density and transfer for selective and efficient SDT. Specifically, metal organic frameworks were constructed through coordination of Fe3+ and ZnTC and simultaneously loading TH to prepare TH@FeM. In infection sites with elevated glutathione, Fe³⁺ is reduced to Fe²⁺, triggering TH@FeM decomposition and TH/ZnTC self-assembly through π-π stacking and electrostatic interactions. IEF from ZnTC to TH drives the formation of S-scheme TH/ZnTC heterojunctions and greatly promotes efficient separation and transfer of the generated charges at the matched interface for efficient generation of reactive oxygen species. Meanwhile, GSH-reductive releases of Fe2+ enable high Fenton reaction activity for chemodynamic therapy. After intravenous injection into a mouse pyomyositis model, the enhanced penetration and retention in the infected muscles implements up to 3.1-folds higher fluorescence intensities than those of the major tissues. Ultrasonication of TH@FeM fully destructs bacteria, downregulates inflammatory factor levels, promotes angiogenesis, and accelerates healing of infected muscles without significant pathological and functional changes in the main organs, leading to continuous decreases in clinical scores and full survival of pyomyositis mice. Thus, the concise design represents the first attempt to explore biofilm-responsive heterojunction formation for synergistic chemo-/sonodynamic therapies of bacterial infections.
Statement of significance
Nearly 80 % of chronic infections are linked to biofilm formation on living tissues. Extracellular polysaccharides produced by biofilms confer protection, making bacteria 10−1000 times more resistant to antibiotics compared to their planktonic counterparts, thus complicating treatment. Sonodynamic therapy (SDT) offers promising advantages in addressing antibiotics-resistant bacteria and deep tissue infections, but challenges remain in the development of highly efficient, infection-responsive, and biocompatible sonosensitizers. Herein, we propose biofilm-responsive generation of dual-porphyrin heterojunctions with matched interface and strong interfacial electric field, which enhance charge density and transfer for selective and efficient SDT. The glutathione-responsive formation and charge transfer mechanisms were both theoretically calculated and experimentally validated. Furthermore, target accumulation and treatment efficacy were demonstrated in a pyomyositis model.
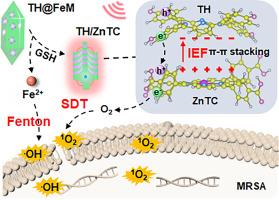
生物膜触发的双卟啉异质结界面组装用于化脓性肌炎的化学/声动力治疗。
声动力疗法(SDT)在对抗抗生素耐药细菌和深部组织感染方面具有优势,但在开发高效,感染反应性和生物相容性的声敏剂方面仍然存在挑战。本文提出了四(4-羟基苯基)卟啉(TH)和四(4-羧基苯基)锌卟啉(ZnTC)构建双卟啉异质结(TH/ZnTC),其匹配的界面和强界面电场(IEF)增强了电荷密度和转移,实现了选择性和高效的SDT。具体而言,通过Fe3+和ZnTC配合,同时加载TH,构建金属有机骨架,制备TH@FeM。在谷胱甘肽升高的感染位点,Fe³⁺还原为Fe 2⁺,通过π-π堆叠和静电相互作用触发TH@FeM分解和TH/ZnTC自组装。从ZnTC到TH的IEF驱动S-scheme TH/ZnTC异质结的形成,极大地促进了所产生电荷在匹配界面上的有效分离和转移,从而有效地生成活性氧。同时,gsh还原性Fe2+的释放使化学动力学治疗具有高芬顿反应活性。经小鼠肌炎模型静脉注射后,感染肌肉的穿透和滞留增强,荧光强度比主要组织高3.1倍。超声TH@FeM可充分破坏细菌,下调炎症因子水平,促进血管生成,加速感染肌肉的愈合,而主要器官的病理和功能没有明显改变,导致化脓性肌炎小鼠临床评分持续下降,完全存活。因此,简洁的设计代表了首次尝试探索生物膜响应异质结的形成,以协同化疗/声动力治疗细菌感染。重要意义:近80%的慢性感染与活组织上的生物膜形成有关。生物膜产生的胞外多糖具有保护作用,使细菌对抗生素的抵抗力比浮游生物高10-1000倍,从而使治疗复杂化。声动力疗法(SDT)在解决抗生素耐药细菌和深部组织感染方面具有很好的优势,但在开发高效、感染反应性和生物相容性的声敏剂方面仍然存在挑战。在此,我们提出生物膜响应生成具有匹配界面和强界面电场的双卟啉异质结,以提高电荷密度和转移,实现选择性和高效的SDT。理论计算和实验验证了谷胱甘肽响应形成和电荷转移机制。此外,在化脓性肌炎模型中证实了靶积累和治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


