A dual In vitro and In silico approach to evaluate 1,4-naphthoquinone-1,2,3-triazole hybrids against atovaquone-resistant malaria
IF 1.6
4区 医学
Q3 PARASITOLOGY
引用次数: 0
Abstract
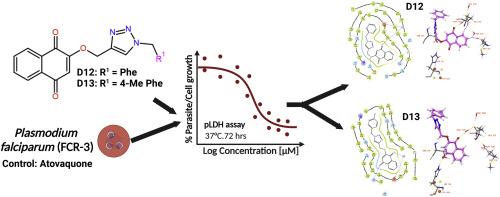
Malaria continues to pose a major global health burden affecting millions annually. Despite advancements in treatment, the emergence of drug-resistant Plasmodium strains has undermined current treatment strategies, including atovaquone. Atovaquone is a key mitochondrial inhibitor targeting the cytochrome bc1 (cyt bc1) complex, with resistance primarily driven by mutation in the cytochrome b gene. Moreover, atovaquone's reliance on a single target site and its pharmacokinetic limitations further underscore the urgent need for alternative drugs. To address these challenges, this dual in vitro and in silico study evaluated ten 1,4-naphthoquinone-1,2,3-triazole hybrids targeting atovaquone-resistant (FCR3) P. falciparum. Molecular modelling studies were performed on Saccharomyces cerevisiae (PDB ID 3CX5), involving the building of a mutant model to simulate the Y279S mutation (equivalent to Y268S mutation in P. falciparum), in order to rationalise the observed results. Additionally, pharmacokinetic properties and drug-likeness of these hybrids were predicted in silico. Hybrids D12 and D13 exhibited strong antiplasmodial activities, 61- and 52-fold, respectively, more than atovaquone. Molecular modelling studies indicated a strong correlation between in silico and in vitro activities by displaying binding interactions between the ligand and the mutant model. Structure-activity relationships (SAR) analysis identified key structural features essential for favourable binding interactions with target binding site residues. Furthermore, in silico evaluations of these hybrids suggested good oral bioavailability and high gastrointestinal absorption, with no significant risk of severe toxicity. Hybrids D12 and D13 exhibit potential as lead candidates, with their strong in vitro efficacy well-supported by in silico data, warranting further optimisation and development.
体外和计算机双方法评价1,4-萘醌-1,2,3-三唑杂种抗阿托伐醌耐药疟疾的作用。
疟疾继续造成重大的全球健康负担,每年影响数百万人。尽管在治疗方面取得了进展,但耐药疟原虫菌株的出现破坏了目前的治疗策略,包括阿托伐醌。Atovaquone是一种针对细胞色素bc1 (cyt bc1)复合物的关键线粒体抑制剂,其耐药性主要由细胞色素b基因突变驱动。此外,阿托伐酮对单一靶点的依赖及其药代动力学的局限性进一步强调了替代药物的迫切需要。为了应对这些挑战,这项体外和计算机双试验评估了10个针对阿托伐醌耐药(FCR3)恶性疟原虫的1,4-萘醌-1,2,3-三唑杂交体。我们对酿酒酵母(PDB ID 3CX5)进行了分子模型研究,包括建立一个突变模型来模拟Y279S突变(相当于恶性疟原虫中的Y268S突变),以使观察到的结果合理化。此外,用计算机预测了这些杂交种的药代动力学性质和药物相似性。D12和D13表现出较强的抗疟原虫活性,分别是阿托伐醌的61倍和52倍。分子模型研究表明,通过显示配体和突变模型之间的结合相互作用,在硅和体外活性之间存在很强的相关性。结构-活性关系(SAR)分析确定了与目标结合位点残基有利的结合相互作用所必需的关键结构特征。此外,这些混合物的计算机评价表明,良好的口服生物利用度和高胃肠道吸收,没有严重毒性的显著风险。杂种D12和D13表现出作为主要候选物的潜力,其强大的体外功效得到了硅数据的支持,值得进一步优化和开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental parasitology
医学-寄生虫学
CiteScore
3.10
自引率
4.80%
发文量
160
审稿时长
3 months
期刊介绍:
Experimental Parasitology emphasizes modern approaches to parasitology, including molecular biology and immunology. The journal features original research papers on the physiological, metabolic, immunologic, biochemical, nutritional, and chemotherapeutic aspects of parasites and host-parasite relationships.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


