The influence of soluble epoxide hydrolase inhibition and their PUFA-derived epoxides in osteoblast bone metabolism: an in vitro study
IF 3.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular and cell biology of lipids
Pub Date : 2025-07-31
DOI:10.1016/j.bbalip.2025.159669
引用次数: 0
Abstract
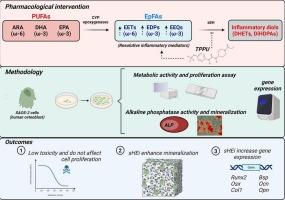
EpFAs are crucial mediators in resolving inflammation and regulating various biological processes. However, their activity is constrained by the rapid metabolism mediated by the soluble epoxide hydrolase (sEH), which converts EpFAs into inactive or even pro-inflammatory diols. Nevertheless, the specific effects of soluble epoxide hydrolase inhibition (sEHI) and EpFAs on osteogenic cell metabolism remain unclear. Cultures of the human immortalized osteoblast-like cell line (SAOS-2) were treated with varying concentrations (0.1–10 μM) of the potent sEHI TPPU or EpFAs (epoxyeicosatrienoic acids [EETs], epoxydocosapentaenoic acids [EDPs], and epoxyeicosatetraenoic acids [EEQs], derived from arachidonic acid [ARA], eicosapentaenoic acid [EPA], and docosahexaenoic acid [DHA], respectively). Cellular metabolic activity and proliferation were evaluated. Osteogenic potential was assessed through alkaline phosphatase activity, mineral nodule formation, and the expression of osteogenic markers, including Runx-2, Osx, Col1, Bsp, Opg, Ocn, Opn, and sEH. Treatment with TPPU and EpFAs enhanced cellular metabolic activity during the first 48 h without affecting proliferation. Alkaline phosphatase activity and mineral nodule formation assays revealed that TPPU significantly stimulated osteogenic differentiation, while EpFAs, particularly EETs, EEQs, and EDPs, promoted osteogenesis predominantly at later stages. Furthermore, TPPU modulated the expression of key osteogenic markers, enhancing differentiation. Notably, EDPs were found to disrupt the synergistic effects between sEHI and EpFAs during the mineralization process. These findings suggest that sEHI enhances mineralization and may facilitate tissue regeneration in vitro. The differential effects of EpFAs and their interplay with sEHI provide insights into potential therapeutic strategies for bone tissue engineering and regeneration.
可溶性环氧化物水解酶抑制及其pufa衍生的环氧化物对成骨细胞骨代谢的影响:体外研究。
epfa是解决炎症和调节各种生物过程的重要介质。然而,它们的活性受到可溶性环氧化物水解酶(sEH)介导的快速代谢的限制,该酶将EpFAs转化为无活性甚至促炎二醇。然而,可溶性环氧化物水解酶抑制(sEHI)和EpFAs对成骨细胞代谢的具体影响尚不清楚。用不同浓度(0.1-10 μM)的强效sEHI TPPU或EpFAs(分别由花生四烯酸[ARA]、二十碳五烯酸[EPA]和二十二碳六烯酸[DHA]衍生的环氧二十碳三烯酸[EETs]、环氧二十二碳五烯酸[EDPs]和环氧二十碳四烯酸[EEQs])处理人永生化成骨样细胞系(SAOS-2)。观察细胞代谢活性和增殖情况。通过碱性磷酸酶活性、矿物结节形成和成骨标志物(包括Runx-2、Osx、Col1、Bsp、Opg、Ocn、Opn和sEH)的表达来评估成骨潜力。TPPU和EpFAs在前48 h内增强了细胞代谢活性,但不影响细胞增殖。碱性磷酸酶活性和矿物结节形成分析显示,TPPU显著刺激成骨分化,而EpFAs,尤其是eet、eeq和EDPs,在后期主要促进成骨。此外,TPPU调节关键成骨标志物的表达,促进分化。值得注意的是,在矿化过程中,EDPs破坏了sEHI和epfa之间的协同作用。这些发现表明,sEHI可以增强矿化,并可能促进体外组织再生。EpFAs的不同作用及其与sEHI的相互作用为骨组织工程和再生的潜在治疗策略提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.00
自引率
2.10%
发文量
109
审稿时长
53 days
期刊介绍:
BBA Molecular and Cell Biology of Lipids publishes papers on original research dealing with novel aspects of molecular genetics related to the lipidome, the biosynthesis of lipids, the role of lipids in cells and whole organisms, the regulation of lipid metabolism and function, and lipidomics in all organisms. Manuscripts should significantly advance the understanding of the molecular mechanisms underlying biological processes in which lipids are involved. Papers detailing novel methodology must report significant biochemical, molecular, or functional insight in the area of lipids.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


