Effect of cytokine TGF-β1 on pathological changes in diabetic retinopathy
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The purpose of the study was to determine the role of the cytokine transforming growth factor beta 1 (TGF-β1) in the progression of diabetic retinopathy and to assess its importance as a potential therapeutic target for slowing down pathological changes in the retina. To achieve this goal, scientific publications selected from leading international databases were analysed. The studies investigated the molecular mechanisms of TGF-β1 activation, including Smad-dependent and Smad-independent pathways, such as MAPK, PI3K/Akt, Wnt/β-catenin and NF-κB, which play a key role in fibrotic, angiogenic and inflammatory processes. Particular attention was paid to the interaction of TGF-β1 with vascular endothelial growth factor (VEGF), which promotes the formation of new pathological vessels with increased permeability, as well as the regulation of the extracellular matrix. The analysis revealed that TGF-β1 is a key regulator of structural changes in the retina under hyperglycaemia, affecting basement membrane thickening, disruption of the blood-retinal barrier, increased vascular permeability and the development of chronic inflammation. It has been shown that elevated levels of TGF-β1 contribute to the progression of fibrosis by activating the epithelial-mesenchymal transition of retinal pigment epithelial cells, which leads to tissue remodelling. In addition, it was found that activation of TGF-β1 under the influence of hyperglycaemia is associated with increased oxidative stress, inflammatory reactions and angiogenesis. The analysis of experimental studies in animal models where streptozotocin was used to induce hyperglycaemia, as well as clinical observations of patients with diabetic retinopathy, allowed to assess the effectiveness of TGF-β1 inhibitors in reducing fibrotic changes, angiogenesis, and chronic inflammation. The results obtained contribute to the formation of a theoretical basis for the further development of therapeutic strategies aimed at inhibiting TGF-β1 as an important component of the pathogenesis of diabetic retinopathy.
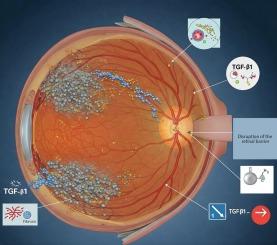
细胞因子TGF-β1对糖尿病视网膜病变病理改变的影响。
本研究的目的是确定细胞因子转化生长因子β1 (TGF-β1)在糖尿病视网膜病变进展中的作用,并评估其作为减缓视网膜病理变化的潜在治疗靶点的重要性。为了实现这一目标,从主要的国际数据库中挑选的科学出版物进行了分析。研究探讨了TGF-β1激活的分子机制,包括smad依赖性和smad非依赖性通路,如MAPK、PI3K/Akt、Wnt/β-catenin和NF-κB,这些通路在纤维化、血管生成和炎症过程中发挥关键作用。特别关注TGF-β1与血管内皮生长因子(VEGF)的相互作用,促进新的病理血管形成,通透性增加,并调节细胞外基质。分析发现,TGF-β1是高血糖状态下视网膜结构变化的关键调控因子,影响基底膜增厚、血视网膜屏障破坏、血管通透性增加和慢性炎症的发生。已有研究表明,TGF-β1水平升高通过激活视网膜色素上皮细胞的上皮-间质转化,导致组织重塑,从而促进纤维化的进展。此外,我们发现在高血糖的影响下,TGF-β1的激活与氧化应激、炎症反应和血管生成的增加有关。通过对链脲佐菌素诱导高血糖动物模型的实验研究分析,以及对糖尿病视网膜病变患者的临床观察,可以评估TGF-β1抑制剂在减少纤维化改变、血管生成和慢性炎症方面的有效性。本研究结果为进一步开发抑制TGF-β1作为糖尿病视网膜病变发病机制重要组成部分的治疗策略奠定了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


