Computational Multiscale Modeling of Pulsed Field Ablation Considering Conductivity and Damage Anisotropy Reveals Deep Lesion Morphologies
Abstract
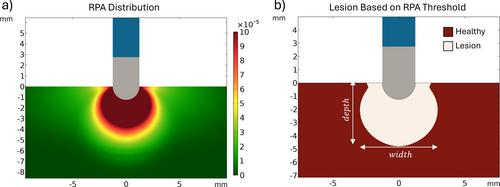
Pulsed Field Ablation (PFA) is an electroporation-based treatment modality to perform cardiac tissue ablations. Heart parenchyma is mainly constituted by elongated myocytes organized in fibers. This anisotropic morphology results in a preferential pathway for the electric current to flow along. Assuming conventional PFA modeling approaches in which lesions form where the electric field surpasses a threshold, such conductance anisotropy would result in relatively wide and shallow lesion morphologies when PFA applications are delivered with a focal monopolar catheter. Contrary to that, some recent preclinical data present narrow and deep elongated lesions. This study presents a multiscale simulation approach able to estimate electroporation treatment outcomes when applied in a highly anisotropic tissue such as the myocardium. In this work, a microscopic model was first implemented mimicking the conformation of the cardiac tissue. Longitudinal and transversal electric fields at different frequencies and magnitudes were applied to characterize the expected anisotropic behavior at the tissue level in terms of electric conductivity and expected membrane disruption due to electroporation. Second, the microscopic characterization was integrated into a macroscopic model of a focal ablation catheter in contact with the myocardial tissue to simulate the delivery of monopolar PFA treatments. The microscopic simulations results show that when low electric field magnitudes are applied, the induced membrane disruptions predominantly appear in fibers parallel to the electric field. However, at higher field magnitudes, a demarcated superior sensitivity is observed in perpendicular orientation. The integration of these anisotropic properties into the macroscopic model predicts width/depth ratios of 1.2 compared to the ratios of about 2 predicted with conventional modeling. In this work, the presented multiscale model and approach can predict relatively narrow and deep lesions, as observed preclinically.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


