FMO2 expression confers cardioprotection in doxorubicin therapy while preserving antitumor activity
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
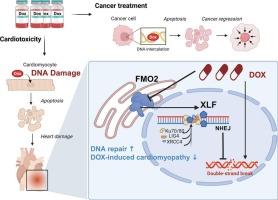
Doxorubicin (DOX) is a widely used anthracycline chemotherapeutic agent, but its clinical application is limited by severe side effects, particularly DOX-induced cardiomyopathy (DIC) which is closely associated with oxidative stress, DNA damage and, subsequent apoptosis. Flavin-containing monooxygenase 2 (FMO2), a cardiac-enriched enzyme, catalyzes NADPH-dependent oxidative metabolism of diverse pharmaceuticals. Our previous work demonstrated that FMO2 expression confers cardioprotective effects against ischemic cardiomyopathy; however, the role of FMO2 in DIC has not been demonstrated.
Methods
DIC was induced in wild-type, FMO2−/−, and cardiomyocyte-specific FMO2-overexpressing mice. Neonatal rat ventricular myocytes were assessed following adenoviral-mediated FMO2 knockdown or overexpression. Transcriptome profiling and chromatin analysis elucidated the mechanism involving FMO2-mediated attenuation of DOX-induced DNA damage. A xenograft model was used to evaluate the impact of FMO2 on DOX's antitumor efficacy.
Results
FMO2 expression was suppressed in heart following DIC. Genetic ablation of FMO2 exacerbated DIC, whereas cardiomyocyte-specific FMO2 overexpression mitigated DOX-induced cardiac injury. Mechanistically, FMO2 reduced DOX-induced DNA damage by stabilizing chromatin-associated X-ray repair cross-complementing protein 4-like factor (XLF), thereby promoting DNA repair. Furthermore, FMO2 expression did not compromise DOX's antitumor efficacy.
Conclusions
FMO2 expression confers cardiac protection against DIC by stabilizing chromatin-associated XLF to facilitate DNA repair. Critically, cardiac FMO2 expression preserves DOX's antitumor efficacy, establishing it as a potential target for DIC management.
FMO2表达在阿霉素治疗中赋予心脏保护作用,同时保持抗肿瘤活性
多柔比星(DOX)是一种广泛使用的蒽环类化疗药物,但其严重的副作用限制了其临床应用,特别是DOX诱导的心肌病(DIC)与氧化应激、DNA损伤和随后的细胞凋亡密切相关。含黄素单加氧酶2 (FMO2)是一种心脏富集酶,可催化多种药物的nadph依赖性氧化代谢。我们之前的研究表明,FMO2表达对缺血性心肌病具有心脏保护作用;然而,FMO2在DIC中的作用尚未得到证实。方法在野生型、FMO2−/−和心肌细胞特异性FMO2过表达小鼠中诱导dic。在腺病毒介导的FMO2敲低或过表达后,对新生大鼠心室肌细胞进行了评估。转录组分析和染色质分析阐明了fmo2介导的dox诱导DNA损伤衰减的机制。采用异种移植模型评价FMO2对DOX抗肿瘤效果的影响。结果DIC后心肌组织fmo2表达受到抑制。FMO2基因消融加重DIC,而心肌细胞特异性FMO2过表达减轻dox诱导的心脏损伤。机制上,FMO2通过稳定染色质相关x射线修复交叉互补蛋白4样因子(XLF)减少dox诱导的DNA损伤,从而促进DNA修复。此外,FMO2的表达并不影响DOX的抗肿瘤效果。结论sfmo2表达通过稳定染色质相关的XLF促进DNA修复,对DIC具有保护作用。关键是,心脏FMO2表达保留了DOX的抗肿瘤功效,使其成为DIC治疗的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


